|
International Steering Committee for Black Carbon Reference Materials |
|
-- Thank you for visiting -- |

Go To BC Standard Recommendations
![]()
The
term black carbon (BC) is a general one applied to various carbonaceous products of
incomplete combustion and includes chars, charcoals and
soots. BC is ubiquitous in the
environment, including in aerosols, sediments and soils. Current
intercomparison efforts have made clear the need for a suite of widely
available and representative BC benchmark materials. To address this need, an
international steering committee was formed during the 1999 Geochemical
Society meeting (Goldschmidt Conference). This committee is charged with
developing representative and accessible BC reference materials for the
environmental sciences community.
In
May 2000 this committee issued preliminary recommendations for BC materials
spanning the combustion continuum. Recommendations include i) five matrices
containing BC materials, such as soot, charcoal, aerosol, soil, sediment, and
ii) an additional small set of materials potentially creating BC during
analysis, for use in detecting methodological artifacts, such as humics,
kerogens and coals. This collection has to be chosen to balance a number of
competing demands. Materials must be: 1) generally available, 2) homogeneous,
3) stable over a longer period of time, 4) inexpensive to obtain, and 5)
represent natural samples. After issuing preliminary recommendations via this
web site, the BC steering committee will actively solicit input from
scientists and will use this information to make a final set of
recommendations of BC benchmark materials.
Michael
W. I. Schmidt, Co-chair (Physical Geography, Univ. Cologne,
Germany)
Carrie
Masiello
Co-chair (Lawrence Livermore National Laboratory, USA)
William
P. Ball
(Johns Hopkins University, USA)
Lloyd
Currie
(NIST, USA)
Jan
O. Skjemstad
(CSIRO Adelaide, Australia)
Dwight
M. Smith
(University of Denver, USA)
All of us
can be reached at BC-steer@bgc-jena.mpg.de, which is the address to which we
invite you to send comments and recommendations.
![]()
Proposed
Reference Materials:
![]()
BC And Matrices Containing Black Carbon
We suggest a set of five reference materials, i.e. soot-BC, charcoal-BC, and three matrices (soil, sediment, aerosol) containing BC
![]()
Five Materials Potentially Interfering With BC Analysis
![]()
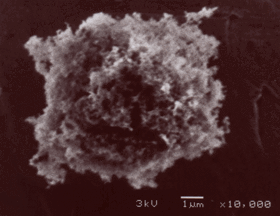
Black carbon
(1,2) soot particles are nucleated, grow and are deposited from the gas phase
during combustion. They have surface, structural and compositional
characteristics demonstrably different from those of commonly-used surrogates
for atmospheric BC. Some of these surrogates are carbon blacks which have been
post-treated with NOx (thus increasing adsorption), or graphite,
for example. These materials differ in significant ways from environmentally
produced BC. Major differences include soot-BC particle reactivity, hydration,
radiative properties, C,H,O content, surface functionalities, unpaired
electron spin density, surface area, and porosity. The properties of
commercial carbons are sufficiently different from those of environmental BC
that we recommend against their use in BC studies. Instead, we recommend using
a soot standard produced in the laboratory from saturated hydrocarbons.
N-hexane is recommended as the fuel, both because of a large amount of soot
characteristic and reactivity data already in the scientific literature (e.g.
2-6) and because of variabilities due to fuel and combustion conditions.
Careful control of air/fuel ratio in premixed flames recently has resulted in
the establishment of quantitative relationships between several key particle
properties(6). Test quantities of standard soot can be prepared from an
established protocol in individual laboratories, or batches contracted from
such commercial carbon producers as Cabot, Degussa, Columbian, etc., following
a specified protocol. A limited number of one-gram quantities of n-hexane
soot, prepared under controlled conditions and having specific properties, are
available for test purposes from D.M. Smith, Department of Chemistry and
Biochemistry, University of Denver, Denver, CO 80208 (dwismith@du.edu).
References:
1) T. Novakov, The role of soot and primary oxidants in atmospheric chemistry,
Sci. Total Environ. 36, 1-10 (1984). 2) E.D. Goldberg, “Black Carbon in The
Environment”, Wiley, New York, 1985. 3) M.S. Akhter, A.R. Chughtai, and D.M.
Smith, The structure of hexane soot I: Spectroscopic studies, Appl. Spectrosc.
39(1), 143-153 (1985). 4) H. Cachier, Carbonaceous combustion aerosols, in “Atmospheric
Particles”, R.M. Harrison and R.E. vanGrieken, eds., Wiley, Chichester, New
York, 1998. 5) A.R. Chughtai, G.R.
Williams, M.M.O. Atteya, N.J. Miller, and D.M. Smith, Carbonaceous particle
hydration, Atmos. Environ. 33, 2679-2687 (1999). 6) A.R. Chughtai, J. Kim, and
D.M. Smith, The effect of air/fuel ratio on properties and reactivity of
combustion soots, J. Atmos. Chem., 2002, in press.
Commercial
charcoals and other lignocellulosic chars represent the carbonized residues of
initial starting plant material that has been subjected to incomplete
combustion and/or pyrolysis. The chemical and physical properties of charcoal
prepared from lignocellulosic material in the laboratory vary significantly
and are dependent on multiple factors, including starting material,
temperature, ramping rates, atmosphere, and oxygen mass transfer conditions
(e.g. Mackay and Roberts, 1982). In this context, we propose that either a
large quantity of a natural char be obtained and/or that synthetic char be
obtained using reproducible techniques from a single reproducible source.
Moreover, because it still can be difficult to ensure reproducible results, we advocate the batch production of a single large quantity of “ reference material” if possible. At the time of this writing, the logistical and financial support for production, storage and shipping of large quantities of lignocellulosic char is under negotiation. If this issue is resolved, we will advocate the production of single large batches of each of two well-homogenized charred materials, one of which is representative of wood char and one of which is representative of grass char. Either of these could be 1) synthesized from well-defined biomass sources under standardized conditions of temperature, temperature ramp rate and gas flow (details of which are yet to be fully established), or 2) culled and homogenized from naturally burned material in as standard a means as possible (e.g., from wood logs of established size from a known tree species, grown at a given location). For example, the charring of selected forest hardwoods under controlled optimal conditions of temperature and wood humidity may represent a simple and inexpensive source of char for homogenization. We recommend rice straw as a reasonable starting material for grass char, since it is easy to obtain, inexpensive, and high in Si (a major chemical difference between grass and wood). We suggest that such char standards be well homogenized, stored in a controlled environment, and shipped on request, after careful splitting of aliquots. Alternatively, if a sufficient quantity of natural material can be obtained and homogenized, and if sufficient scientific interest can be generated, it may be possible that some group or agency could obtain the funding needed to establish and maintain a standard material. The Committee would strongly advocate such an approach.
Reference: Mackay D. M., Roberts, P. V. 1982. The dependence of char and carbon yield on lignocellulosic precursor composition, Carbon, 20 (2), 87-94.
We suggest the inclusion of two soils high in char and preferably common in the US, Australia and Eurasia. We suggest a Pellustert from Australia. These soils, commonly containing large amounts of charred organic carbon (30 % of the organic carbon present), and in the mm size range, as well a German Chernozem will be included. German Chernozems developed some 10,000 years ago from loess and are much younger soils than those developed in Australia. Chernozems contain large amounts of charred organic carbon (almost 50 % of the organic carbon present), according to the UV-oxidation NMR method, and the suggested soil was characterized in some detail (soil number 6 in the paper (1) by Schmidt et al. 1999). In the case of the Pellustert we suggest the 0-20 cm horizon and in the 25-50 cm (Axh) horizon for the Chernozem. In both cases, about 50 kg have been sampled, dried, sieved <2mm, homogenized, sterilized with gamma radiation, and 80g subsamples are available for distribution on request from Jan Skjemstad, CSIRO Land and Water, PMB 2, Glen Osmond, SA 5064, Australia.
References:
1) Schmidt, Skjemstad, Gehrt, Kögel-Knabner 1999. Charred organic carbon in
German chernozemic soils. European Journal of Soil Science 50 (2), 351-365. 2)
Skjemstad, Taylor, Smernik 1999. Estimation of charcoal (char) in Soils.
Communication in soil science and Plant analysis 30 (15&16), 2283-2298.
A standard reference material from marine sediments is currently available from the National Institute of Standards and Technology (NIST). Organics in marine sediment standard reference material (SRM) 1941a is a marine material collected from the coastal marine environment. This material is intended for use in evaluating analytical methods for the determination of selected polycyclic aromatic hydrocarbons, polychlorinated biphenyl congeners, chlorinated pesticides and sulphur in marine particulate material and similar matrices. The certificate and ordering information are available at ww.nist.gov/srm/
A standard
reference material that exemplifies atmospheric particulate matter also is
currently available from NIST. Urban Dust Standard Reference Material (SRM)
1649a is an atmospheric particulate material collected in the Washington DC
urban area in 1976-1977, using a baghouse specially designed for this purpose.
The particulate material was collected over a period in excess of 12 months,
and therefore represents a time-integrated sample. While the sample is not
intended to be representative of the area in which it was collected, it should
generally typify atmospheric particulate matter obtained from an urban area.
This material is intended for use in evaluating analytical methods for the
determination of selected polycyclic aromatic hydrocarbons (PAH),
polychlorinated biphenyl congeners, chlorinated pesticides, and total carbon
in atmospheric particulate material and similar matrices. Analytical results
for the black carbon component, determined as “Elemental Carbon",
clustered at about 13 g, 49g and 81g of elemental carbon per kg of material,
giving evidence of method dependence. This SRM also has information and/or
reference values for natural C- 14 in several chemical fractions, including
elemental carbon and certain PAH. The NIST-Standard SRM 1649a is available as
a powder, but small (research) quantities have been prepared on quartz
filters. The certificate and ordering information can be found at www.nist.gov/srm/.
![]()
Five Materials Potentially Interfering With BC Analysis
![]()
We also suggest a set of five reference materials (shale, two coals, natural organic matter from a river, and melanoidin) which could potentially interfere with the analysis of BC. All materials, except for the melanoidin, could contain charcoal, or degradation products of fire residues.
The U.S. Geological Survey reference Green River Shale (SGR-1) was collected from the Mahogany zone of the Green River Formation. It is a petroleum and carbonate-rich shale (Ctot 28 mass%, Cinorg 3.2 mass%). At the time of preparation, shale oil tests yielded 51 to 57 gallons per ton. Element concentrations were determined by cooperating laboratories using a variety of analytical methods. Certificate values are based primarily on international data compilations (Abbey, 1983, Gladney and Roelandts, 1988, Govindaraju, 1994). Initial USGS studies (Flanagan, 1976) provide limited background information on this reference material. Material can be ordered through: Dr. Stephen A. Wilson, U.S. Geological Survey, Box 25046, MS 973, Denver, CO 80225, USA. Info: http://minerals.cr.usgs.gov/geo_chem_stand/shale.html
More than 1000g of Suwannee River natural organic matter (NOM) were isolated using reverse osmosis technology, then H+-saturated, freeze dried, and homogenized. This sample contains only 7%, and represents a 92.9 % recovery of, dissolved organic carbon. The NOM sample is available for 20 USD per 100mg. The elemental composition of dry Suwannee River NOM is: 48.8 %C; 3.9 %H; 39.7 %O; 1.02 %N; 0.60 %S; 0.02 %P; 7.0 %Ash (Total 101.0%). A brief description of the sampling and isolation procedure can be found on the web. Orders to: International Humic Substances Society, Dr. Paul R. Bloom, Department of Soil, Water, and Climate, University of Minnesota, 1991 Upper Buford Circle, St. Paul, MN 55108, USA, e-mail: ihss@soils.umn.edu, Info: http://www.ihss.gatech.edu/sales.html
Melanoidins are an excellent proxy for a class of organic materials likely to interfere with BC separations. Melanoidins were defined first by Maillard, 1917 (Annales de chimie (Paris) 7: 113-152). A synthesis protocol also has been published: Allard et al. 1997 (Organic Geochemistry 26: 691-703) and Olsson et al. 1978 (Acta Chem. Scandinavica B32: 249-256). A batch of 20 kg can be produced according to the following protocol (Allard et al., 1997): amino acids / glucose (1/9, mass ratio), 4 amino acids: glutamic acid, aspartic acid, glycine, leucine. mixture dissolved in HCl 6N, under reflux for 24h, insoluble melanoidins are isolated by centrifugation or filtration, then rinsed with acetone and dried.
We suggest
two coal samples representing typical end-members in coal rank, a lignite and
low-volatile bituminous coal. Anthracites were not included because of their
low reactivity and relative scarcity. As an indicator of coal rank, the
lignite (Beulah-Zap) has a low reflectance (0.25) whereas the bituminous coal
(Pocahontas) has a higher reflectance (1.68). The bituminous coal contains
about 10% of inertinite, suspected to represent (at least partly) fossil
charcoal. No inertinite values are available for the lignin
standard. Subsamples can be purchased for 5 USD per 5 g from the Argonne National Laboratory USA (Assistant Controller, OCF/201, Argonne National Laboratory, 97000 South Cass Avenue, Argonne, IL 60439, USA, web: http://www.anl.gov/PCS Characteristics. Mass % C, H, O values on moisture and ash free basis, S and ash are on the dry basis. 4 - Pocahontas C: 91 / H 4.4 / O 2 / S 0.7 /ash 5; 5 - Beulah-Zap C 73 / H 4.8 / O 20 / S 0.8 / ash 10.
Appendix to BC Reference Materials :
Descriptive information concerning the NIST Standard Reference Material Program, including the Catalog and Certificates of Analysis, may be found at: www.nist.gov/srm/. This web address has links to all categories of information. Particularly useful in the catalog are Table 109.2 (Organic Constituents), and Table 111.7 (Soils, Sediments, and Sludges). Potentially relevant materials in Table 109.2 are SRMs 1649a (urban dust), 1941b (organics in marine sediment), 1944 (New York-New Jersey waterway sediment), and 2975 (diesel particulate matter). Table 111.7 has in addition SRMs 1646a (estuarine sediment), 2709 (San Joaquin soil), 2711 (Montana soil), and 8704 (Buffalo River sediment). Two of these SRMs (1649a and 1941a) have been noted above. Some of the environmental reference materials listed in the tables include concentration values for total (non-carbonate) carbon, but SRM 1649a is the only one that includes values for "elemental carbon" (information values) and C-14 (information and reference values). Selected references, drawn from the Certificate of Analysis for SRM 1649a are below. The final certificate was issued on 31 January 2001, and is posted on the NIST website which also specifies the price (208 USD) and unit of issue (2.5g).
References:
[3] Schantz,
M.M., Benner, B.A. Jr., Hays, M.J., Kelly, W.R., Vocke, R.D., Jr., Demiralp,
R., Greenberg, R.R., Schiller, S.B., Lauenstein, G.G., and Wise, S.A.,
"Certification of Standard Reference Material (SRM) 1941a, Organics in
Marine Sediment”, Fresenius J. Anal. Chem. 352, pp. 166-173, (1995). [6]
Wise, S.A., Schantz, M.M., Hays, M.J., Koster, B.J., Sharpless, K.S, Sander,
L.C., Benner, B.A., Jr., and Schiller, S.B., "Certification of Polycyclic
Aromatic Hydrocarbons in Mussel Tissue and Air Particulate Standard Reference
Materials”, Polycyclic Aromat. Compd. 9, pp. 209-216, (1996). [26] L.A.
Currie, B.A. Benner, Jr., H. Cachier, R. Cary, J.C. Chow, E.R.M. Druffel, T.I.
Eglinton, O. Gustafsson, P.C. Hartmann, J.I. Hedges, J.D. Kessler, T.W.
Kirchstetter, D.B. Klinedinst, G.A. Klouda, J.V. Marolf, C.A. Masiello, T.
Novakov, A. Pearson, K.M. Prentice, H. Puxbaum, J.G. Quinn, C.M. Reddy, H.
Scmid, J.F. Slater, J. Watson, and S.A. Wise, "A Critical Evaluation of
Interlaboratory Data on Total, Elemental and Isotopic Carbon in the
Carbonaceous Particle Reference Material, NIST SRM 1649a”, submitted (2001).
[30] Klouda, G.A., Klinedinst, D.D., Steel, E.B., Benner, B.A., Jr., and
Parish, H.J., "Exploring a Method to Produce an Urban Dust Particle
Filter Standard”, J. Aerosol Sci. 27, Suppl. 1, pp. S351-S352, (1996). [42]
Currie, L.A., Klouda, G.A., Benner, B.A. Garrity, K., and Eglinton, T.I.,
"Isotopic and Molecular Fractionation in Combustion; Three Routes to
Molecular Marker Validation, Including Direct Molecular ‘Dating’ (GC/MS)”,
Atm. Environ. 33, pp. 2789-2806, (1999). [43] Currie, L.A., Eglinton, T.I.,
Benner, B.A., Jr., and Pearson, A., "Radiocarbon Dating of Individual
Chemical Compounds in Atmospheric Aerosol: First Results Comparing Direct
Isotopic and Multivariate Statistical Apportionment of Specific Polycyclic
Aromatic Hydrocarbons”, Nucl. Instrum. Methods Phys. Res. B123, pp. 475-486,
(1997). [44] Christopher M. Reddy, Ann Pearson, Li Xu, Ann P. McNichol, Bruce
Benner,Jr., Lloyd A. Currie, and Timothy I. Eglinton, "Molecular Isotopic
Characterization of Polycyclic Aromatic Hydrocarbons in Standard Reference
Materials”, submitted to Environ. Sci. Technol. (2001). [45] C.A. Masiello,
E.R.M. Druffel, and L.A. Currie, "Radiocarbon Measurements of Black
Carbon in Aerosols and Ocean Sediments”, Geochim. Cosmochim. Acta, 2001, in
press.
Recent And Current Developments
Several oral
and poster presentations on the need for BC standards and the Steering
Committee recommendations have been given, including the following:
5th
International Conference on Methods and Applications of Radioanalytical
Chemistry, 9-14 April 2000, Kailua-Kona, Hawaii, USA
Gordon
Research Conference on Organic Geochemistry, 13-18 August 2000, New Hampshire,
USA
7th
International Conference on Carbonaceous Particles in the Atmosphere, 26-29
November 2000, San Juan, Puerto Rico
20th
International Meeting on Organic Geochemistry, 10-14 September 2001, Nancy,
France
Symposium
on Reference Materials for Ocean Sciences, Ocean Studies Board, U.S. National
Academy of Sciences, 5-7 September 2001, Islamorada, Florida, USA
A
comparative analysis resulting from the 1999 Goldschmidt Conference now has
been published:
M.W.I.
Schmidt, J.O. Skjemstad, C.I. Czimczik, B. Glaser, K.M. Prentice, Y. Gelinas,
and T.K. Kuhlbusch, Comparative analysis of black carbon in soils, Global
Biogeochemical Cycles 15, 163-167 (2001).
Revised 10/24/01