Atmospheric Radiation
Contents
- Introduction
- Thermal Radiation
- The Greenhouse Effect
- The Earth's Energy Budget
- The Radiative Environment
- Spectra of the Greenhouse Gases
- The Global Warming Debate
- References
Introduction
Thermal Radiation
Thermal motion of the charged particles in matter causes electromagnetic radiation, called thermal radiation from its cause, though it is no different from other electromagnetic radiation. This implies that matter will also absorb electromagnetic radiation. In a closed system, emission and radiation will equilibrate at some temperature T. Since the emission and absorption properties do not depend on the arrangments, these properties will hold also when there is no equilibrium. A sample of matter at temperature T in space will cool gradually to 0K if there is no radiation present to absorb.
The emissivity e of a surface is the energy radiated per unit area per unit time, with units W/m2. It is usually assumed that the radiation is Lambertian, or diffuse. The absorptivity a of a surface is the fraction of incident radiation absorbed by the surface, also assumed to be Lambertian. The assumption of diffuse emission and absorption is not fundamental; it just makes the discussion simpler. Both e and a may be functions of the frequency or wavelength of the radiation. By considering radiation exchange in thermal equilibrium, Kirchhoff demonstrated that e/a was a universal constant at any frequency and temperature equal to the emissivity of a perfect absorber, or black body, with a = 1. That is, a good absorber is also a good emitter.
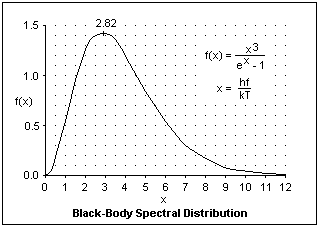 The spectrum of thermal radiation is illustrated at the right. The abscissa is the ratio x = hf/kT = hc/λkT, proportional to the frequency. hf is the quantum energy of radiation of frequency f, or wavelength λ = c/f, and kT is the average thermal energy per equivalent harmonic oscillator (two "degrees of freedom," one for kinetic energy and one for potential energy). k is Boltzmann's constant, the gas constant per molecule. The amount of radiation decreases rapidly at both small and large frequencies with a maximum at x = 2.82. This remarkable and useful formula was discovered by Max Planck, and was the beginning of quantum theory.
The spectrum of thermal radiation is illustrated at the right. The abscissa is the ratio x = hf/kT = hc/λkT, proportional to the frequency. hf is the quantum energy of radiation of frequency f, or wavelength λ = c/f, and kT is the average thermal energy per equivalent harmonic oscillator (two "degrees of freedom," one for kinetic energy and one for potential energy). k is Boltzmann's constant, the gas constant per molecule. The amount of radiation decreases rapidly at both small and large frequencies with a maximum at x = 2.82. This remarkable and useful formula was discovered by Max Planck, and was the beginning of quantum theory.
The maximum of the curve occurs at x = 2.82, or hf = 2.82kT, from which we can find that λT = 5.102 x 106 nm-K. This is the peak of the emission per unit frequency interval. Since frequency and wavelength intervals are related by df = - dλ/λ2, the energy density per unit wavelength interval is proportional to x5/(ex - 1), where x = hc/λkT. The maximum of this curve is at x = 4.96, which gives λmaxT = 2.901 x 106 nm-K. The rule that λmaxT = constant is called Wien's Law, in either case. The maximum depends on which expression for the spectrum that you are using, and really has no absolute significance by itself, but is only useful for comparison. Although the wavelength interval result is more often quoted, the frequency interval result may be more meaningful. For solar radiation at 5750 K, the maxima are at 505 nm and 887 nm, respectively.
If a is independent of frequency, then the total energy radiated per unit area per unit time is W = σT4, which is Stefan's Law. This relation was known long before Planck's formula, and is a consequence of classical thermodynamics. The value of σ was determined in terms of universal constants by Boltzmann. In calorie units, it is 8.14 x 10-11 cal/cm2-min-K4. In SI, it is 5.6705 x 10-8 W/m2-K4.
The Greenhouse Effect
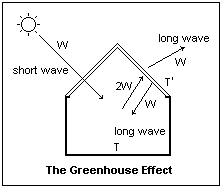 The greenhouse effect is illustrated at the right with a diagram of a greenhouse, a building with a glass roof. Sunlight, 6000K short-wave radiation, mostly passes through the glass bringing an energy flux of W watt. The interior of the greenhouse emits radiation at a much lower temperature, say 300K, which is long-wave radiation. Assume the glass absorbs long-wave radiation completely (which is nearly a fact). When a steady state is reached, the glass is brought to a temperature T' at which it radiates an energy flux of W watt to the exterior. The same energy flux is re-radiated to the interior of the greenhouse, so the interior of the greenhouse must radiate an energy flux of 2W to the glass. This increased energy flux means that the interior must reach a temperature T > T' sufficient for this. In this way, the interior of the greenhouse assumes a higher temperature than it otherwise would if it absorbed W watt of short-wave radiation and re-emitted W watt of long-wave radiation.
The greenhouse effect is illustrated at the right with a diagram of a greenhouse, a building with a glass roof. Sunlight, 6000K short-wave radiation, mostly passes through the glass bringing an energy flux of W watt. The interior of the greenhouse emits radiation at a much lower temperature, say 300K, which is long-wave radiation. Assume the glass absorbs long-wave radiation completely (which is nearly a fact). When a steady state is reached, the glass is brought to a temperature T' at which it radiates an energy flux of W watt to the exterior. The same energy flux is re-radiated to the interior of the greenhouse, so the interior of the greenhouse must radiate an energy flux of 2W to the glass. This increased energy flux means that the interior must reach a temperature T > T' sufficient for this. In this way, the interior of the greenhouse assumes a higher temperature than it otherwise would if it absorbed W watt of short-wave radiation and re-emitted W watt of long-wave radiation.
Many simplifying assumptions have been made here, such as neglecting radiative transfer with the environment at some lower temperature T" <' T', but the general mechanism is clear. Per square metre, W = σT4, if we assume the emissivity is 1. Actually, it is sufficient to assume that the emissivity is the same for all surfaces involved, not necessarily 1. Then, W = σT'4, and 2W = σT4. When the energy leaving the glass equals that absorbed, σ(T4 - σT'4) = σT'4 = W, or T4 = 2T'4, which means that T = 21/4T' = 1.189 T'. Since the glass must be hotter than its surroundings, the interior of the greenhouse is still hotter. If we know W, then we can find both T' and T.
A qualitative explanation of the terrestrial greenhouse effect may be useful here. The surface of the Earth is heated by short-wavelength solar radiation during the day, while the earth radiates continuously at long wavelengths. The equilibrium temperature of the Earth is determined by the equality of these energy fluxes, with the input constant and the output determined by the temperature of the Earth. The average surface temperature of the Earth is about 288K. If the atmosphere were transparent to infrared radiation, the radiation to space would exceed the solar input, and the Earth would cool. Equilibrium would be reached at a surface temperature of about 217K, or about -70°F. There would then be no liquid water, nor life, on the Earth.
However, convective processes establish a nearly linear decrease of atmospheric temperature from 288K at the surface to 217K at around 12 km altitude. The boundary temperatures are determined by radiation, but the lapse rate is not. This includes the densest part of the atmosphere, which will be the only part giving a significant contribution to radiative transfer, and in which water vapor is present in significant concentration. The water vapor absorbs long-wavelength radiation very strongly, and will be nearly opaque to it, so that it is emitted and absorbed continuously. The radiation will be emitted at the surface at a temperature of 288K, but will be emitted into space at 217K, as required for equilibrium. Water vapor is by far the most important contributor to this process, by means of its large concentration and strong rotational spectrum.
Carbon dioxide has a strong band near 15μ which is very well placed for greenhouse activity, another band at a shorter wavelength that is less important, and no rotational spectrum at longer wavelengths, since it does not have a permanent dipole moment. However, the 15μ band covers only a very limited part of the spectrum, and carbon dioxide is not present in large concentration (less than 400 ppm), so carbon dioxide will have a much smaller effect than water vapor. It should be realized that both the greenhouse effect and carbon dioxide are essential to life on Earth.
We can apply this to the earth as follows. The earth absorbs the short-wave sunlight falling on its projected disc of area πR2, and radiates long-wave radiation from its surface area 4πR2. The long-wave radiation per unit area must then be 1/4 the short-wave radiation incident per unit area. The incident short-wave radiation is about 1.94 cal/cm2-min, which works out to 1353 W/m2. Let's assume that half of this is absorbed by the earth, or 676 W/m2. The flux that must be radiated by every square metre is a quarter of this, or 169 W/m2, presuming that the earth reaches a uniform temperature. This is not nearly as bad an assumption as would be imagined, since the earth is actually at about the same temperature all over, thanks to the oceans and atmosphere. If the long-wave emissivity is assumed to be unity, also not a bad assumption, then 169 = σT4, or T = 235K or -38°C, if the atmosphere were transparent to long-wave radiation. This is not far from the truth.
The atmosphere is nearly transparent to short-wave radiation, but is relatively opaque to long-wave radiation, except in a few "windows" of transmission. Therefore, it acts like the glass of a greenhouse, absorbing and emitting long-wave radiation according to its temperature. Since it must emit the same amount of radiation to space that we assumed in the preceding paragraph, its effective temperature must be T' = -38°C. We now use our greenhouse theory to estimate the ground temperature at 1.189T' = 279K or +6°C. This is sufficient to melt the water and allow life to exist, as it does.
It is remarkable that our approximate estimate is close to actuality. The accurate calculation of the radiation balance of the atmosphere is extremely difficult because of the variability of the earth's surface, the differences in cloud cover, and other factors. In fact, in spite of computers, only simplified models are practical, and there is really not a good handle on this important factor, just enthusiastic scientists waving papers in the air and shouting at each other. The general effect has been well-known for a considerable time. Fortunately for us, it does not depend too greatly on the concentration of the trace gases responsible for the long-wave absorption, of which water vapor is the most important, and also quite variable.
If we think of greenhouses with multiple glass roofs, then each roof will contribute a factor 1.189, and pretty soon you are talking real insulation. Another glass roof to the earth would give a surface temperature of 332K or 59°C, which is downright uncomfortable. Three would give 122°C, and water would boil. It is not clear to me that carbon dioxide, with its absorption in closely limited bands, could ever be responsible for such extreme conditions.
The Earth's Energy Budget
The earth generates heat internally, and this internal source drives plate tectonics, the magnetic field, volcanic eruptions and earthquakes. Some energy also comes in the fast protons and electrons of the solar wind, which cause interesting ionospheric and auroral effects, and there is even a little from cosmic rays, which are mainly very energetic protons and photons. All of these sources of energy are inconsequential compared to the copious bath of radiation in sunlight, which extends from 0.15 μm in the far ultraviolet to 4 μm in the infrared. We can, therefore, safely neglect these inputs and concentrate on the solar input. The total power received on a surface normal to the direction of the sun outside the atmosphere at the earth's distance is 1.94 cal/cm2-min or 1353 W/m2. The spectrum is close to a black-body spectrum for 6000K, crossed by narrow absorption lines due to absorption in the chromosphere of the sun, the Fraunhofer lines.
As observed at the surface, direct sunlight, which is about 27% of that incident, has been modified by atmospheric absorption and scattering. The energy at wavelengths shorter than 0.29 μm has been cut off due to the creation of ozone and its strong absorption. However, the atmosphere is remarkably transparent to the remainder of the spectrum, only a few weak lines due to oxygen (the diatomic molecule) and water vapor being evident. Scattering is stronger at shorter wavelengths, so the scattered radiation is noticeably blue, and makes the blue of the sky, while the longer wavelengths remain in the direct beam. This scattering is from density fluctuations in the upper atmosphere, not from individual molecules as is sometimes asserted.
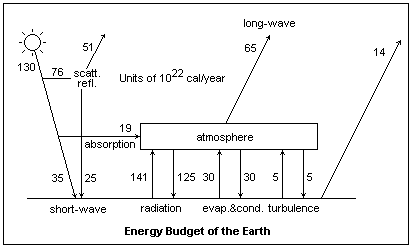 An estimate of the steady-state energy budget of the earth is shown at the left. The numbers represent energy in units of 1022 cal/year. The figures are rough estimates, but illustrate the relative magnitudes of the contributions. The sun provides 130 in the form of short-wave radiation (0.15 to 4.0μm) at the top of the atmosphere. Of this, 76 is scattered or reflected, and 19 is absorbed, leaving 35 to reach the surface as direct radiation. The absorbed portion includes interaction with ozone, and this energy heats the upper atmosphere. Of the scattered and reflected light, 25 also reaches the surface, while 51 is radiated into space as short-wave radiation. This makes the albedo of the earth to be about 0.39.
An estimate of the steady-state energy budget of the earth is shown at the left. The numbers represent energy in units of 1022 cal/year. The figures are rough estimates, but illustrate the relative magnitudes of the contributions. The sun provides 130 in the form of short-wave radiation (0.15 to 4.0μm) at the top of the atmosphere. Of this, 76 is scattered or reflected, and 19 is absorbed, leaving 35 to reach the surface as direct radiation. The absorbed portion includes interaction with ozone, and this energy heats the upper atmosphere. Of the scattered and reflected light, 25 also reaches the surface, while 51 is radiated into space as short-wave radiation. This makes the albedo of the earth to be about 0.39.
The atmosphere absorbs 141 from the surface, and re-emits 125 to the surface as long-wave (4-120μm) radiation. The net amount, 65, is radiated to space. The atmosphere receives 30 by condensation of water, and gives up 30 by evaporation, most of which is part of the hydrologic cycle. The surface receives 5 by turbulence, and releases the same amount of energy on the average by the same means. Finally, 14 is directly radiated from the earth to space through the "window" in the infrared spectrum from 8 - 12μm, and in the near infrared below 4 μm.
This diagram suggests the complexity of making an energy budget for the earth, because of the many things that must be taken into consideration. Dynamic changes are even more difficult to predict, because of the interrelation between factors. It shows the very important role of radiation in the energy budget, and the different parts played by short-wave and long-wave radiation.
The Radiation Environment
The solid and liquid surfaces of the earth are usually good absorbers and good radiators. An exception are cloud and fresh snow surfaces, where light is repeatedly reflected without absorption, and returned little reduced in intensity. The reflection may be 70-80%, and these surfaces appear white to the eye. Rock and vegetation reflect only 10%-30%. Short-wave radiation largely penetrates water, where it is absorbed almost totally. Only the part that is reflected at the upper surface, which depends on the angle of incidence, is not absorbed. The earth and the oceans appear dark from space, with a blue haze from scattering and white clouds making a definite contrast. The average cloud cover is about 52%, it is said.
Things are quite different in the long-wave spectrum. Here, water has strong absorption from 4 - 8 μm, and beyond 25 μm, so that now clouds and snow are black, not white. Snow and thick clouds radiate like black bodies at long wavelengths, so exchange of energy by radiation is very important. Clouds may be warmed by radiation from below, while they are cooled at their tops by radiation into space. This creates instability (a large lapse rate) so thunderstorms can continue to boil during the night. Liquid water, interestingly, strongly absorbs everything except the visible. It is even blacker with long waves, but the energy does not penetrate as far as short waves will, so absorption and radiation of infrared takes place only in superficial layers. Cooling is rapid at the surface, which may produce the convection that keeps the surface waters well mixed.
Spectra of the Greenhouse Gases
The discussion of the greenhouse effect showed that the absorption of long-wave radiation in the atmosphere was important to its explanation. The major constituents of the atmosphere, the diatomic gases nitrogen and oxygen, and the 1% of argon, do not interact with electromagnetic radiation in the long-wave spectrum at all. The only trace gases present in significant amounts are water vapor and carbon dioxide. Water vapor has a permanent dipole moment, and so a strong pure rotation spectrum beginning at about 25 μm and extending with greater and greater absorption to longer wavelengths. It also has a vibration-rotation band for the bending mode at around 6.3 μm, and for an asymmetric stretching mode at 2.66 μm.
The pure rotation spectrum of carbon dioxide is at much too long wavelengths to play a role, but there is a strong bending mode band at 14.7 μm, as well as an asymmetric stretching vibration at 4.26 μm. The 14.7 μm band is at a critical wavelength, and has a considerable effect in spite of the low concentration of carbon dioxide. Carbon dioxide must be considered in atmospheric radiative transfer, but it is much less important than water.
It is usual to express absorption in terms of Beer's Law, which is the integrated form of dI/dx = -Iadx, I = Ioe-ax, where a is the linear absorption coefficient. Sometimes it is convenient to use the optical density, which is the product of the density and the distance, d = ρx, expressed in g/cm2. Then, if a' = a/ρ, I = Ioe-a'd. The advantage is that a' is not a function of the density and distribution of the absorber, only on the number of absorber molecules present.
Beer's Law is almost useless for expressing the absorption of radiation in the atmosphere. The spectra consist of sharp, separated absorption lines. If you start with a uniform radiation spectrum, first the centres of the lines are absorbed and removed from the beam, then the wings of the lines, and in a complex way that depends on the details of the spectrum (which for a long time were not accurately known). Attempts to use an average absorption, as if the atmosphere were a "grey" body failed utterly. This is a place where computers can help greatly when a detailed absorption curve is available, and many good calculations have been carried out. We won't go into this in detail here, only point it out lest it be thought that things were straightforward and easy.
The Global Warming Debate
There is evidence that the average temperature of the Earth is increasing. The rate of increase is slow, and is certainly not unreasonable. The average temperature has fluctuated rather widely in recent geological history. In fact, it is generally assumed that we are in an interglacial era, and that the temperature is changing is less remarkable than if it remained unchanged. The reasons for continental glaciation are still quite unknown, and prediction is not possible. It is somewhat remarkable that permanent ice still persists at polar latitudes and high altitudes, since this does not appear to be typical in geologic history. At present, then, it would be reasonable for the Earth's temperature either to decrease or to increase, since it is at a rather intermediate level, perhaps cooler than normal, so an increase would not be surprising.
The argument current among some scientists, politicians and the general public (not remarkable for geologic knowledge) is that the increase in temperature is caused by carbon dioxide emitted into the atmosphere by human activity, and that restriction of coal burning by electrical utilities, together with some less effective measures, will reduce the carbon dioxide concentration and solve the problem. It is indeed an inconvenient truth that this simple argument is rubbish.
We have noted above that by far the most effective greenhouse gas is water vapor. Some very small increase in its atmospheric concentration, perhaps caused by human activity, would also cause an increased greenhouse effect, and an increase in the Earth's average temperature if the greenhouse effect is indeed responsible for climate. Exactly the same argument can be made for water vapor as for carbon dioxide. For example, burning natural gas produces large quantities of the principal greenhouse gas, while if coal is burned to produce the same amount of heat, only the much less effective carbon dioxide is emitted, turning the usual argument on its head. The atmosphere is no more a closed system for water vapor than it is for carbon dioxide, and what is added may not end up in the atmosphere after all. In fact, agriculture could be responsible for much water vapor, and since agriculture increases at the same rate as population, this would provide an anthropogenic source as well.
Not only is water vapor not mentioned in connection with global warming, neither is the effect of population, except peripherally. If global warming is anthropogenic, then the only means of preventing it would be a significant reduction in human numbers, which seems politically impossible. It is another inconvenient truth that there appears to be no way for human population to be self-limiting until resources are exhausted and starvation does the job. Russia seems to be the only major country expecting a decrease in population (which they are doing all possible to avoid). This is valid even in the carbon dioxide picture. Predictions are now being made for times when the population will certainly exceed the resources, as soon as 2050, when the population will (hypothetically) have doubled. How much limitation of carbon dioxide can be realized in this case?
More carbon dioxide and warmer weather are good news for plants (they survive and give us food even with the small amount of carbon dioxide available in the atmosphere). Such conditions are maintained in some actual greenhouses to increase crop yield, but any positive consequences of global warming or increased carbon dioxide are extremely unpopular with the enthusiasts.
None of the proposals for controlling climate can be expected to have any measurable effects whatever, as good as they may be for conservation and efficiency.
References
E. W. Hewson and R. W. Longley, Meteorology Theoretical and Applied (New York: John Wiley & Sons, 1944).
F. A. Berry, Jr., E. Bollay and N. R. Beers, eds., Handbook of Meteorology (New York: McGraw-Hill, 1945).
Return to Weather Index
ID="Postscript">Composed by J. B. Calvert
Created 29 May 2007
Last revised 21 July 2009
 The spectrum of thermal radiation is illustrated at the right. The abscissa is the ratio x = hf/kT = hc/λkT, proportional to the frequency. hf is the quantum energy of radiation of frequency f, or wavelength λ = c/f, and kT is the average thermal energy per equivalent harmonic oscillator (two "degrees of freedom," one for kinetic energy and one for potential energy). k is Boltzmann's constant, the gas constant per molecule. The amount of radiation decreases rapidly at both small and large frequencies with a maximum at x = 2.82. This remarkable and useful formula was discovered by Max Planck, and was the beginning of quantum theory.
The spectrum of thermal radiation is illustrated at the right. The abscissa is the ratio x = hf/kT = hc/λkT, proportional to the frequency. hf is the quantum energy of radiation of frequency f, or wavelength λ = c/f, and kT is the average thermal energy per equivalent harmonic oscillator (two "degrees of freedom," one for kinetic energy and one for potential energy). k is Boltzmann's constant, the gas constant per molecule. The amount of radiation decreases rapidly at both small and large frequencies with a maximum at x = 2.82. This remarkable and useful formula was discovered by Max Planck, and was the beginning of quantum theory. The greenhouse effect is illustrated at the right with a diagram of a greenhouse, a building with a glass roof. Sunlight, 6000K short-wave radiation, mostly passes through the glass bringing an energy flux of W watt. The interior of the greenhouse emits radiation at a much lower temperature, say 300K, which is long-wave radiation. Assume the glass absorbs long-wave radiation completely (which is nearly a fact). When a steady state is reached, the glass is brought to a temperature T' at which it radiates an energy flux of W watt to the exterior. The same energy flux is re-radiated to the interior of the greenhouse, so the interior of the greenhouse must radiate an energy flux of 2W to the glass. This increased energy flux means that the interior must reach a temperature T > T' sufficient for this. In this way, the interior of the greenhouse assumes a higher temperature than it otherwise would if it absorbed W watt of short-wave radiation and re-emitted W watt of long-wave radiation.
The greenhouse effect is illustrated at the right with a diagram of a greenhouse, a building with a glass roof. Sunlight, 6000K short-wave radiation, mostly passes through the glass bringing an energy flux of W watt. The interior of the greenhouse emits radiation at a much lower temperature, say 300K, which is long-wave radiation. Assume the glass absorbs long-wave radiation completely (which is nearly a fact). When a steady state is reached, the glass is brought to a temperature T' at which it radiates an energy flux of W watt to the exterior. The same energy flux is re-radiated to the interior of the greenhouse, so the interior of the greenhouse must radiate an energy flux of 2W to the glass. This increased energy flux means that the interior must reach a temperature T > T' sufficient for this. In this way, the interior of the greenhouse assumes a higher temperature than it otherwise would if it absorbed W watt of short-wave radiation and re-emitted W watt of long-wave radiation. An estimate of the steady-state energy budget of the earth is shown at the left. The numbers represent energy in units of 1022 cal/year. The figures are rough estimates, but illustrate the relative magnitudes of the contributions. The sun provides 130 in the form of short-wave radiation (0.15 to 4.0μm) at the top of the atmosphere. Of this, 76 is scattered or reflected, and 19 is absorbed, leaving 35 to reach the surface as direct radiation. The absorbed portion includes interaction with ozone, and this energy heats the upper atmosphere. Of the scattered and reflected light, 25 also reaches the surface, while 51 is radiated into space as short-wave radiation. This makes the albedo of the earth to be about 0.39.
An estimate of the steady-state energy budget of the earth is shown at the left. The numbers represent energy in units of 1022 cal/year. The figures are rough estimates, but illustrate the relative magnitudes of the contributions. The sun provides 130 in the form of short-wave radiation (0.15 to 4.0μm) at the top of the atmosphere. Of this, 76 is scattered or reflected, and 19 is absorbed, leaving 35 to reach the surface as direct radiation. The absorbed portion includes interaction with ozone, and this energy heats the upper atmosphere. Of the scattered and reflected light, 25 also reaches the surface, while 51 is radiated into space as short-wave radiation. This makes the albedo of the earth to be about 0.39.