Preface
The purpose of this series, and an introduction to the electron
These pages, or units, are a review of electronics based on laboratory experiments, with little purely theoretical work or comprehensive treatment of any topic. Nevertheless, most of the fundamental concepts of electronics are discussed and illustrated. The emphasis is on understanding. This is not a collection of electronics projects, and there is no emphasis on construction practices, though some construction may be interesting and valuable. There is no use of SPICE-like circuit calculations, which can also be informative, but is no substitute for prototyping and experimentation. An effort has been made to include interesting historical electronics, such as vacuum tubes and Nixie displays, which also furnishes a deeper understanding of the fundamentals. References are given to further information.
There is more here than can be put into a typical electronics course of 30 or 40 lectures, which should concentrate on the fundamentals. These are the nature of amplification (transistors, for example), feedback, stability, relaxation oscillators and phase-locked loops.
The author has taught electronics at university level, and has a rather low opinion of most of the courses and texts available. This is mainly the consequence of the instructors being instructors and researchers, not electronic engineers in anything but name, often leaving the laboratory in charge of a subordinate of limited knowledge and experience. The laboratory itself is often managed in that wonderful orderly fashion that makes it of limited usefulness and little fascination, characterized by set experiments and "writeups" that teach little, as well as by physical surroundings that are nothing like a real workbench. For learning electronics, however, the laboratory is absolutely essential. The only way to handle a laboratory, incidentally, is to assign to each student his own, lockable, bench. The way not to handle it is to "set up" benches in the laboratory in advance of each "experiment" and otherwise to pack everything away in a single storeroom.
I know very little of the quality of electronics instruction in high schools and trade schools, but I expect it is no better than in universities, and possibly the less said about it, the better. Actually, it has probably disappeared in American schools. In any case, its benefits will depend on the qualities of the individual instructor, qualities that are not valued by the educational establishment, like intelligence, knowledge and skill.
As a hobby, electronics has much to recommend it. It is useful, clean, safe, educational and even inexpensive, considering other hobbies. Unlike chemistry, it will not attract the attention of the authorities. In general, hobbyists have much to recommend them over university professors, although they generally err on the other end of the scale, being deficient in theory and often holders of fixed, though erroneous, ideas. Electronics is best thought of as a semi-professional hobby, where some application is in mind, and is certainly essential for radio or electronic control. A complete workbench can be assembled for around $2000, including 100 MHz scope, and a large assortment of components and tools. Interesting work can be done for less than $200, however, with only a digital multimeter (DMM) and a breadboard--details are given in another unit.
Like all hobbies with a scientific, technical or skilled mechanical foundation, in recent years electronics and amateur radio have withered in the United States, while seeming to retain vitality in Europe. It has become much more difficult to exercise such hobbies, but it is still possible to some degree, and there seems to be a nucleus of intelligent amateurs to sustain them. This trend has been noticed by others. For example, look at "R.I.P. for D.I.Y.", in Scientific American, May 2002, p. 26. Scientific American, for its part, killed The Amateur Scientist column some time ago, and it disappeared like any mathematics in this advertising-heavy and increasingly light journal. These are not trivial trends--they are already being reflected in the declining technological knowledge of the American public, and in the loss of skilled craftsmanship. In fact, Scientific American would be more aptly titled Nonmathematical American these days, and the term "Scientific American" considered for an honored place in the Halls of Oxymoron. A recent issue had not one equation, and only two graphs, one illustrating scientific plagiarism, and the other the number of genomes decoded. Two articles, at least, mentioned things that have never been done as all but everyday events (detecting gravitational waves and making a quantum computer). The publishers no doubt know their market well.
To use and understand this material, the reader should already know DC circuits and how to use a DMM (digital multimeter), how to use algebra and trigonometry and some calculus. A university course in electronics could also be helpful, but do not expect too much from it. The mathematics is absolutely essential, and anyone wanting to understand electronics should acquire skills in algebra, trigonometry and calculus. There is no way around this. Mathematical modelling works exceptionally well in electronics, and is always relied upon whenever possible.
When I began this series in the summer of 2001, I intended only to review some fundamentals and useful circuits. However, it tended to grow and branch out as interesting possibilities presented themselves. I hoped to include a brief look at vacuum tubes, but this expanded greatly when I discovered I could obtain a great variety of tubes and demonstrate their interesting features. This is an exellent supplement for those who know transistors, greatly broadening the understanding. I thought experiments with cathode-ray tubes and photomultipliers were not feasible, but both subjects became possible at reasonable cost, and I was finally able to build circuits that were impossible for me when the devices were still normally available. Some subjects may be repeated in later units, usually with a different emphasis and applications.
The units are written after I repeat the experiments mentioned yet again, with any improvements I can devise, and the actual results are stated. All circuits have been tested in the laboratory, and are not simply figments of the imagination. I cannot emphasize too strongly the benefits of actual testing, even of circuits that seem straightforward and simple. The presentation is in a form that seems most informative to me: just having to write it up is good practice, and reveals gaps in knowledge that have to be filled. I have done it as a comprehensive review for my own purposes. It is not designed for any particular audience, and if it helps you, fine. If not, there are many, many other places to learn electronics. Remember, you didn't have to pay anything for it! Best of luck and success.
James B. Calvert, Denver, Colorado, 2001
Electrons
Electronics is so-called from the light, negatively-charged elementary particle the electron, which is the usual carrier of energy in all electronic phenomena. The charge of the electron is -1.602 x 10-19 coulomb, and its mass is 9.10 x 10-31 kg. Despite its small size, the effects it produces on a macroscopic scale are very much like those of a particle, and individual electrons can even be perceived (indirectly). On a microscopic scale, the electron exhibits wave properties, and excellently illustrates quantum mechanics. The fact that the charge is negative is a purely arbitrary convention, resulting from historical accidents.
The discrete nature of matter first became evident in chemistry, with the laws of combination and the effects of electrolysis. 96,490 coulombs of charge, a Faraday, produced one mole of univalent substance, showing that there was a great amount of electrical charge in matter, although it was precisely balanced to show no net effect on a macroscopic scale, except in very special circumstances. Electrical forces are, in fact, responsible for the structure of matter, so it is no wonder that electrolysis occurs. The chemist G. Johnstone Stoney (1826-1911) realized that electric charge must be discrete as early as 1874, and in 1891 gave the elementary unit the name of electron. Since Avogadro's number was not well known at the time, his estimate of the electronic charge was inaccurate, but the important thing was the realization of the discreteness of charge. Of course, he had no concept of the electron as a material particle, only as a unit of charge.
The Greek word hlektron referred both to the semi-precious ornament amber, and to an alloy of gold and silver, approximately 80 Au, 20 Ag, that was used in early coinage because of the difficulty of separating gold and silver. In the latter meaning, it has become "electrum." It was the meaning as amber that gave the word "electricity" first to the phenomena of static electricity and later to the whole field. Amber, when rubbed, develops a surface charge and will attract light objects. It happens to be exactly the same as "electron," but purely by accident.
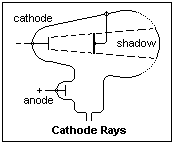 Faraday studied the flow of current through low-pressure gases in 1838, and described the dark space known by his name at the cathode end of the discharge, but could not reach pressures below about 1 torr. The first electrical discharge through low-pressure gases seems to have been created by A. Masson in 1853, when he discharged a Ruhmkorff (induction) coil through a Torricellian vacuum (the space above the mercury in a barometer). Julius Plücker (1801-1868) and Heinrich Geissler (1814-1879) created discharge tubes still often known by their names, in many shapes and colors, as entertaining displays that were the basis of the neon lights of advertising. In these tubes, the light was the result of the rarefied gases through which the electricity passed. W. Hittorf (1824-1914) of Münster studied discharges at lower and lower pressures, discovering the dark space near the cathode that is known by his name in 1869, noting that it became wider, the lower the pressure. When a vacuum was approached, the glows disappeared, but there was still something there, since the glass bulbs fluoresced, as if under the influence of invisible rays proceeding in straight lines from the cathode. This had first been observed by Plücker in 1858. E. Goldstein named them "cathode rays" in 1876, and they were suspected of being ether rays like light. Cathode rays, like light, cast shadows, as illustrated at the right.
Faraday studied the flow of current through low-pressure gases in 1838, and described the dark space known by his name at the cathode end of the discharge, but could not reach pressures below about 1 torr. The first electrical discharge through low-pressure gases seems to have been created by A. Masson in 1853, when he discharged a Ruhmkorff (induction) coil through a Torricellian vacuum (the space above the mercury in a barometer). Julius Plücker (1801-1868) and Heinrich Geissler (1814-1879) created discharge tubes still often known by their names, in many shapes and colors, as entertaining displays that were the basis of the neon lights of advertising. In these tubes, the light was the result of the rarefied gases through which the electricity passed. W. Hittorf (1824-1914) of Münster studied discharges at lower and lower pressures, discovering the dark space near the cathode that is known by his name in 1869, noting that it became wider, the lower the pressure. When a vacuum was approached, the glows disappeared, but there was still something there, since the glass bulbs fluoresced, as if under the influence of invisible rays proceeding in straight lines from the cathode. This had first been observed by Plücker in 1858. E. Goldstein named them "cathode rays" in 1876, and they were suspected of being ether rays like light. Cathode rays, like light, cast shadows, as illustrated at the right.
Sir William Crookes (1832-1919) was led to the study of cathode rays by his work on high vacuum, connected with accurate weighing in the determination of the atomic weight of Thallium. His experiments began in 1873, and in 1875 resulted in the invention of the radiometer, in which vanes are made to rotate when light falls upon them. The cause of rotation was eventually determined to be the collisions of molecules of the rarefied gas, not light pressure. In such a roundabout way he was brought to the study of electrical discharges at low pressures, in which he repeated much of Hittorf's work, which was unknown to him. The Hittorf Dark Space is also known as the Crookes Dark Space. He showed that the rays were deflected by electric and magnetic fields (which seems to have been a fairly common observation). Crookes spoke of "radiant matter," using a term introduced by Faraday, without a very clear understanding of what it might be. In 1895 Jean Perrin (1870-1942) showed that the cathode rays were deflected by a magnetic field as if they were negative charges, which suggested that they were streams of particles.
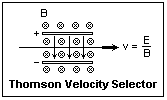 In 1897, J. J. Thomson (1856-1940) of the Cavendish Laboratory at Cambridge University sent a beam of cathode rays through perpendicular electric and magnetic fields, and found to his surprise that all had about the same velocity. The Thomson velocity selector is shown at the right. All particles (of either sign) of the velocity E/B go straight through; others are deflected to one side or the other. Fields of 100,000 V/m and 1.0 T, for example, are required for velocities of 107 m/s, corresponding to acceleration through a voltage difference of 284 V. With the velocity known, electric or magnetic deflection alone served to determine the ratio of charge to mass, e/m, which was found to be very large, 1.76 x 1011 C/kg (Thomson initially obtained about half this value, but it was later corrected). This meant that the particles, called by Thomson "corpuscles," were 1/1837 the mass of a hydrogen atom, assuming that the hydrogen nucleus had an equal positive charge. However, the mass of the hydrogen atom, or, what is the equivalent, Avogadro's number, was still not accurately known. It was remarkable that all cathode rays, now firmly regarded as corpuscles, had the same e/m, regardless of the gas involved in the discharge. Thomson made the deduction that all cathode rays were identical, and composed of the discrete charges proposed by Stoney. For this good reason, Thomson is called the "discoverer of the electron" although, as we see, others were involved. The absolute identity of all electrons is a fundamental principle of physics, hard to understand on a classical basis, but demonstrated excellently in quantum mechanics.
In 1897, J. J. Thomson (1856-1940) of the Cavendish Laboratory at Cambridge University sent a beam of cathode rays through perpendicular electric and magnetic fields, and found to his surprise that all had about the same velocity. The Thomson velocity selector is shown at the right. All particles (of either sign) of the velocity E/B go straight through; others are deflected to one side or the other. Fields of 100,000 V/m and 1.0 T, for example, are required for velocities of 107 m/s, corresponding to acceleration through a voltage difference of 284 V. With the velocity known, electric or magnetic deflection alone served to determine the ratio of charge to mass, e/m, which was found to be very large, 1.76 x 1011 C/kg (Thomson initially obtained about half this value, but it was later corrected). This meant that the particles, called by Thomson "corpuscles," were 1/1837 the mass of a hydrogen atom, assuming that the hydrogen nucleus had an equal positive charge. However, the mass of the hydrogen atom, or, what is the equivalent, Avogadro's number, was still not accurately known. It was remarkable that all cathode rays, now firmly regarded as corpuscles, had the same e/m, regardless of the gas involved in the discharge. Thomson made the deduction that all cathode rays were identical, and composed of the discrete charges proposed by Stoney. For this good reason, Thomson is called the "discoverer of the electron" although, as we see, others were involved. The absolute identity of all electrons is a fundamental principle of physics, hard to understand on a classical basis, but demonstrated excellently in quantum mechanics.
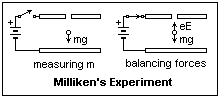 Determination of the charge on the electron was the next order of business. This was done by balancing the gravitational and electric forces on small charged droplets of an aerosol. The mass of a droplet was determined by measuring its rate of fall under gravity alone. Since electric forces are much greater than gravitational forces, the disparity between the small charge of the electron and the large mass of a droplet was reduced, and the method proved practical. Robert A. Millikan (1868-1953) improved the methods of Thomson and his co-workers, and in 1906 published good figures for the electronic charge, which were steadily improved. Now, the mass of an electron could be determined accurately, together with Avogadro's number and the mass of the hydrogen atom. The knowledge of the nature of the electron has been essential to the later development of electronics.
Determination of the charge on the electron was the next order of business. This was done by balancing the gravitational and electric forces on small charged droplets of an aerosol. The mass of a droplet was determined by measuring its rate of fall under gravity alone. Since electric forces are much greater than gravitational forces, the disparity between the small charge of the electron and the large mass of a droplet was reduced, and the method proved practical. Robert A. Millikan (1868-1953) improved the methods of Thomson and his co-workers, and in 1906 published good figures for the electronic charge, which were steadily improved. Now, the mass of an electron could be determined accurately, together with Avogadro's number and the mass of the hydrogen atom. The knowledge of the nature of the electron has been essential to the later development of electronics.
Electrons have many other interesting properties. The apparent mass increases as the speed approaches the speed of light, demonstrating relativistic mechanics. Their lightness also makes quantum-mechanical effects apparent. Electrons have been diffracted like waves, and associate in pairs to produce superconductivity, a macroscopic quantum state. They have an angular momentum, called spin, which gives rise to a magnetic field that is observed in ferromagnetism, where many electrons are all lined up in spin direction. There is a positive electron, a positron, that is antimatter. On meeting an ordinary electron, the pair unite to create photons of electromagnetic energy, the particles disappearing. Conversely, high-energy photons can create electron-positron pairs.
Some of the experiments on vacuum tubes in these pages allow you to observe regions where dense electron beams are moving, but you cannot see the electrons, because they interact only very weakly with visible light, which passes by them without being disturbed.
In this series of pages, semiconductor electronics, gaseous discharges, thermionic emission, vacuum tubes, cathode-ray tubes, photoelectricity and other topics recall the events of the discovery of the electron and its application to technology. For the effects of electric and magnetic fields on electrons, see The Hall Effect. For electrons in metals, see Photoelectricity. For electrons in semiconductors, see Optoelectronics. There is a short discussion of glow discharges in Relaxation Oscillators, in connection with the NE-2 glow lamp. In electronics, we make use almost exclusively of the electric force on the electron, which allows it to transfer energy from one point to another.
You may find Electronics Forum interesting.
Return to Electronics Index
Composed by J. B. Calvert
Created 29 July 2001
Last revised 1 October 2011
 Faraday studied the flow of current through low-pressure gases in 1838, and described the dark space known by his name at the cathode end of the discharge, but could not reach pressures below about 1 torr. The first electrical discharge through low-pressure gases seems to have been created by A. Masson in 1853, when he discharged a Ruhmkorff (induction) coil through a Torricellian vacuum (the space above the mercury in a barometer). Julius Plücker (1801-1868) and Heinrich Geissler (1814-1879) created discharge tubes still often known by their names, in many shapes and colors, as entertaining displays that were the basis of the neon lights of advertising. In these tubes, the light was the result of the rarefied gases through which the electricity passed. W. Hittorf (1824-1914) of Münster studied discharges at lower and lower pressures, discovering the dark space near the cathode that is known by his name in 1869, noting that it became wider, the lower the pressure. When a vacuum was approached, the glows disappeared, but there was still something there, since the glass bulbs fluoresced, as if under the influence of invisible rays proceeding in straight lines from the cathode. This had first been observed by Plücker in 1858. E. Goldstein named them "cathode rays" in 1876, and they were suspected of being ether rays like light. Cathode rays, like light, cast shadows, as illustrated at the right.
Faraday studied the flow of current through low-pressure gases in 1838, and described the dark space known by his name at the cathode end of the discharge, but could not reach pressures below about 1 torr. The first electrical discharge through low-pressure gases seems to have been created by A. Masson in 1853, when he discharged a Ruhmkorff (induction) coil through a Torricellian vacuum (the space above the mercury in a barometer). Julius Plücker (1801-1868) and Heinrich Geissler (1814-1879) created discharge tubes still often known by their names, in many shapes and colors, as entertaining displays that were the basis of the neon lights of advertising. In these tubes, the light was the result of the rarefied gases through which the electricity passed. W. Hittorf (1824-1914) of Münster studied discharges at lower and lower pressures, discovering the dark space near the cathode that is known by his name in 1869, noting that it became wider, the lower the pressure. When a vacuum was approached, the glows disappeared, but there was still something there, since the glass bulbs fluoresced, as if under the influence of invisible rays proceeding in straight lines from the cathode. This had first been observed by Plücker in 1858. E. Goldstein named them "cathode rays" in 1876, and they were suspected of being ether rays like light. Cathode rays, like light, cast shadows, as illustrated at the right. In 1897, J. J. Thomson (1856-1940) of the Cavendish Laboratory at Cambridge University sent a beam of cathode rays through perpendicular electric and magnetic fields, and found to his surprise that all had about the same velocity. The Thomson velocity selector is shown at the right. All particles (of either sign) of the velocity E/B go straight through; others are deflected to one side or the other. Fields of 100,000 V/m and 1.0 T, for example, are required for velocities of 107 m/s, corresponding to acceleration through a voltage difference of 284 V. With the velocity known, electric or magnetic deflection alone served to determine the ratio of charge to mass, e/m, which was found to be very large, 1.76 x 1011 C/kg (Thomson initially obtained about half this value, but it was later corrected). This meant that the particles, called by Thomson "corpuscles," were 1/1837 the mass of a hydrogen atom, assuming that the hydrogen nucleus had an equal positive charge. However, the mass of the hydrogen atom, or, what is the equivalent, Avogadro's number, was still not accurately known. It was remarkable that all cathode rays, now firmly regarded as corpuscles, had the same e/m, regardless of the gas involved in the discharge. Thomson made the deduction that all cathode rays were identical, and composed of the discrete charges proposed by Stoney. For this good reason, Thomson is called the "discoverer of the electron" although, as we see, others were involved. The absolute identity of all electrons is a fundamental principle of physics, hard to understand on a classical basis, but demonstrated excellently in quantum mechanics.
In 1897, J. J. Thomson (1856-1940) of the Cavendish Laboratory at Cambridge University sent a beam of cathode rays through perpendicular electric and magnetic fields, and found to his surprise that all had about the same velocity. The Thomson velocity selector is shown at the right. All particles (of either sign) of the velocity E/B go straight through; others are deflected to one side or the other. Fields of 100,000 V/m and 1.0 T, for example, are required for velocities of 107 m/s, corresponding to acceleration through a voltage difference of 284 V. With the velocity known, electric or magnetic deflection alone served to determine the ratio of charge to mass, e/m, which was found to be very large, 1.76 x 1011 C/kg (Thomson initially obtained about half this value, but it was later corrected). This meant that the particles, called by Thomson "corpuscles," were 1/1837 the mass of a hydrogen atom, assuming that the hydrogen nucleus had an equal positive charge. However, the mass of the hydrogen atom, or, what is the equivalent, Avogadro's number, was still not accurately known. It was remarkable that all cathode rays, now firmly regarded as corpuscles, had the same e/m, regardless of the gas involved in the discharge. Thomson made the deduction that all cathode rays were identical, and composed of the discrete charges proposed by Stoney. For this good reason, Thomson is called the "discoverer of the electron" although, as we see, others were involved. The absolute identity of all electrons is a fundamental principle of physics, hard to understand on a classical basis, but demonstrated excellently in quantum mechanics. Determination of the charge on the electron was the next order of business. This was done by balancing the gravitational and electric forces on small charged droplets of an aerosol. The mass of a droplet was determined by measuring its rate of fall under gravity alone. Since electric forces are much greater than gravitational forces, the disparity between the small charge of the electron and the large mass of a droplet was reduced, and the method proved practical. Robert A. Millikan (1868-1953) improved the methods of Thomson and his co-workers, and in 1906 published good figures for the electronic charge, which were steadily improved. Now, the mass of an electron could be determined accurately, together with Avogadro's number and the mass of the hydrogen atom. The knowledge of the nature of the electron has been essential to the later development of electronics.
Determination of the charge on the electron was the next order of business. This was done by balancing the gravitational and electric forces on small charged droplets of an aerosol. The mass of a droplet was determined by measuring its rate of fall under gravity alone. Since electric forces are much greater than gravitational forces, the disparity between the small charge of the electron and the large mass of a droplet was reduced, and the method proved practical. Robert A. Millikan (1868-1953) improved the methods of Thomson and his co-workers, and in 1906 published good figures for the electronic charge, which were steadily improved. Now, the mass of an electron could be determined accurately, together with Avogadro's number and the mass of the hydrogen atom. The knowledge of the nature of the electron has been essential to the later development of electronics.