Light and Luminescence
Background material important in many applications
The relations between electronics and light have been discussed in a number of pages in this series. Optoelectronics covered mainly the absorption and emission of radiation at PN junctions. Glow discharges were introduced in Relaxation Oscillators as circuit switching devices, and in Vacuum Tubes as voltage regulators and rectifiers. In Phototubes gas and vacuum photocathodes were studied as detectors of radiation, and in Photomultipliers the very sensitive detectors using secondary electron emission were discussed. Electronics is important in the generation and detection of radiation in a great variety of connections, so this page is intended to make some general comments, and to describe experiments not treated elsewhere. In particular, we shall discuss the wide and interesting area of luminescence, in which electronics figures prominently.
As explained in Optoelectronics, the quantum energy of radiation of wavelength λ nm is 1240/λ eV. Energy is absorbed or emitted only in multiples of this amount in the interaction of radiation of a given frequency and matter.
Ultraviolet, Infrared and Visible
Electromagnetic radiation is of the same fundamental nature throughout its frequency spectrum, from frequencies of less than 1 Hz to energetic gamma rays, but the evident properties and means of working with the radiation differ greatly, so the radiation is usually classified on this basis. It happens that our eyes are sensitive to a narrow band of frequencies, and radiation in this band is called light. It has the familiar property of rectilinear propagation, undergoes reflection and refraction, causes no chemical changes (except for the important one of photosynthesis in plants), and is imperceptible to us except through vision. The frequency of this radiation is about 5.4 x 1014 Hz, an almost incomprehensibly rapid oscillation, that is not evident to our senses, and was not realized until Thomas Young measured the wavelength of light by interference early in the 19th century. Wavelengths from 400 to 700 nm (approximately) are characteristic of light.
It is apparent that there is similar radiation both at shorter and longer wavelengths. Our primary natural light source, the Sun, provides only 38% of its energy in light. 53%, more than half, is supplied at longer wavelengths, which makes sunlight a very effective source of warmth. If you have a solar water heater, you are aware of this, and also that the IR also is there on a cloudy day. It is harder to detect the 9% of the energy at shorter wavelengths, but the reddening of sunburn, or erythemal effect, is sometimes painfully evident. These radiations can all be called "light" since they have similar properties. In this case, the term "visible light" is not redundant. That at shorter wavelengths is ultraviolet, while that at longer wavelengths is infrared (once more usually spelled "infra-red"). Both of these, when having the same properties as visible light, may be qualified as "near." The short-wavelength limit of the solar spectrum (at the earth's surface) is about 300 nm, and the long-wavelength limit is about 3900 nm. The "near ultraviolet" is then 300-400 nm, and the "near infrared" 700 to 3900 nm. There are no sharp breaks in properties at any wavelength, and they change smoothly, so there is little use in being more specific. However, some sources specify the visible as 380-780 nm, which goes well beyond what is usually perceptible.
The erythemal effect sets in near the short-wavelength end of the near ultraviolet. Photobiologists distinguish UV-A, 315-400 nm, which is not erythemal, and UV-B, 280-315 nm, which is. Ultraviolet radiation of wavelengths less than 300 nm has quantum energies sufficiently high to disrupt chemical bonds, about 2 eV and higher. A significant dividing point occurs around 200 nm, when oxygen and nitrogen in the air can be dissociated by the radiation, so the air becomes strongly absorbing. Ultraviolet of this and shorter wavelengths no longer can travel through air, so a vacuum must be maintained in spectroscopes and other apparatus for the study and use of this radiation. Radiation from 200-300 nm is often called far ultraviolet, and shorter wavelengths vacuum or extreme ultraviolet. When wavelengths are shorter than 10-40 nm, the properties of ultraviolet merge into the properties of X-rays, and we no longer speak of ultraviolet light, since the means of generation, handling, and detection have become completely different.
The photobiologist's UV-C covers this range of 100-280 nm. In the range 180-220 nm, oxygen is dissocated and forms ozone, which is easily detected by its odor. The range 220-300 nm is fatal to microorganisms (and not very kind to people), so may be called germicidal. The mercury arc has a very strong resonance line at 253.7 nm, in the UV-C, germicidal or far ultraviolet, that has many uses. Note that it is not quite ozone-producing. There is also a strong line at 365 nm, so a mercury arc lamp with a quartz or UV glass envelope can be used as a source of near or far ultraviolet, when provided with a glass filter. An EPROM eraser contains such a lamp, since short-wave UV is required.
We are aware of three common transparent media: air, water and glass, with the property of transmitting light with little absorption. Air is, in fact, quite transparent from around 200 nm in the ultraviolet to the longest wavelengths. Certain gases, such as water vapor or carbon dioxide, absorb in the infrared, usually in bands or regions where the absorption can be considerable, but their effect depends sensitively on their concentration. The principal atmospheric gases, oxygen and nitrogen, do not absorb in the infrared at all. Extreme ultraviolet in the Sun's radiation is absorbed in the upper atmosphere, heating it and causing chemical changes, such as the production of ozone. The ozone is a strong ultraviolet absorber, so little radiation of wavelength shorter than 300 nm reaches the ground. However, any increase in this UV-B ultraviolet has important biological effects.
Water, another common substance, is really a narrow-band filter for visible radiation. While clear and limpid to our eyes, it is opaque to both ultraviolet and infrared. Below the surface, you cannot sunburn, and sunlight is uncommonly cold and unwarming. Above the surface, you get a double dose of ultraviolet, both direct and reflected, and the same with the infrared. You might want to ponder why our eyes are sensitive to precisely those wavelengths that penetrate water, and insensitive to those that do not.
Glass, quartz and transparent plastics are frequently used to transmit light. Ordinary soft crown glass, the usual window glass, transmits some ultraviolet and a good deal of infrared. Typically, glass is transparent down to about 300 nm, so it transmits the UV-A or near ultraviolet. This range is called "black light," used to excite fluorescence in minerals and other substances. Sunlight contains these wavelengths, but the intensity of the sunlight usually masks any evident fluorescence. Paper contains fluorescent sizing to make it whiter in sunlight, as do some laundry detergents and fabric conditioners. A filter can be made that absorbs most of the visible radiation, while letting the 300-400 nm band through, producing striking fluorescent effects in darkness. Plastics generally pass a wider band of wavelengths than glass, and some plastics are notably fluorescent.
Special glasses have been developed that transmit to shorter wavelengths, such as the trade-named Corex (Corning), Uviol (Schott) or Hysil (Chance) glasses, which transmit to beyond 250 nm, including the strong mercury line. Quartz is an excellent ultraviolet transmitter, down to 220 nm or so, but is harder to work and more expensive than glass. On the other end of the spectrum, there are filters that pass IR while blocking visible light, like the dark red plastic at the end of a remote control. Near IR can get into a greenhouse, but far IR from the warmth inside cannot get out. The surface of the earth is warmed in the same way, and kept warm at night. The most important greenhouse gas, by the way, is water vapor.
Eye protection has opposite requirements. Both excessive ultraviolet or infrared are damaging. Intense infrared produces cataract, while ultraviolet results in conjuntivitis. These radiations are especially dangerous when not accompanied by comparably bright visible light, as with IR lasers. Laser infrared radiation can burn the retina without being sensed. Protective goggles should be worn when working with things like glass or open-hearth furnaces, molten iron, or exposed short-wave UV sources, and even in bright sunlight. Ferrous (Fe++) iron gives glass a greenish cast, while absorbing both infrared and ultraviolet. Protex, made by Chance, and similar glasses, pass only visible light, while other glasses cut off at 380 nm or so, and are useful in sunglasses. CuO, and cobalt with ferric (Fe+++) iron, also absorb the infrared strongly. Ferric iron, incidentally, gives a yellow cast to glass. An infrared-absorbing filter is required in transparency projectors, to protect the transparency from the intense condensed light. Incandescent sources are even richer in infrared than sunlight (which is, of course, also incandescent), and are sometimes specifically intended to give heat as well as light ("sunlamps").
Luminescence
Luminescence, as commonly accepted, is the incoherent emission of non-thermal visible light as a result of any sort of excitation, but principally that of radiation of shorter wavelength or the collision of energetic electrons. The concept can be generalized to include the coherent emission of a laser, or an emission at any wavelength, from radio waves to X-rays, after excitation--the transfer of energy--by any means. Thus, it will include excitation by injection of charge carriers, as in LED's, bioluminescence, or indeed luminescence excited by chemical reactions in general, X-rays produced by collision of electrons with a metallic target, light produced by glow discharges and fluorescent lamps, and light produced directly by electric fields, which is called electroluminescence. It is great fun to name and classify these effects, but they are all very similar in principle, though differing greatly in mechanism. The distinguishing principle of luminescence is that the radiation is non-thermal, its spectrum determined by transitions between the energy levels of the luminescent material excited in some determinate way, not by random thermal motion, which produces incandescence. Luminescent radiation may then be called "cold light," in distinction with the "hot light" of incandescence. The Sun is an incandescent source, the Aurora Borealis a luminescent one.
The most characteristic incandescent source is wide-band thermal, or "black-body" radiation, which is fundamentally a noise signal, with a characteristic spectrum that was explained by Max Planck on the basis of thermodynamics and quantum theory. It is called "black-body" because it is typical of radiation from a small opening in a closed body at temperature T, which completely absorbs all radiation falling on it. Real bodies emit and absorb more or less efficiently at various wavelengths, but thermodynamics demands that the ratio of the emissivity to the absorptivity is always the emissivity of a black body, so a good absorber is also a good radiator, and vice versa (Kirchhoff's law). The total radiation of a black body at all wavelengths is proportional to the fourth power of the absolute temperature, E = σT4, the Stefan-Boltzmann law, which can be derived from thermodynamics and Maxwell's result that the pressure of light on a perfectly reflecting surface p = u/3, where u is the energy density of the radiation. The constant σ = 5.67 x 10-8 W m-2 K-4. It would have been very difficult to derive this law empirically, because of the complicated radiative behavior of real bodies.
The emittance of black-body radiation in a wavelength interval dλ is e dλ, where e = (2πhc2/λ5)[exp(hc/λkT) - 1]-1 (Planck's law). The energy density in the volume is 4/c times this value. Here, h is the celebrated Planck's constant, 6.626 x 10-34 J-s, k is Boltzmann's constant, 1.38 x 10-23 J/K, and c is, of course, the speed of light. Black-body radiation can be considered as consisting of random pulses as well as of a superposition of sinusoids, representations which are equivalent because of Fourier's theorem. The spectrum of an actual source may be different, but often closely resembles this, and so can be characterized by a temperature T. Narrow-band filtered thermal radiation has all the characteristics of narrow-band noise, such as a dominant wavelength and a fluctuating amplitude. The clearest distinction between incandescence and luminescence is represented by the difference between black-body radiation and laser radiation.
A useful criterion for distinguishing incandescence and luminescence is to compare the temperature of the radiating system with a "color temperature" based on the spectrum. The distinction is clear with a fluorescent lamp, where the color temperature may be 5000 K (daylight), while the lamp is cool enough to touch. It is also clear with a hot poker, at 1500 K, which has an equivalent color temperature. A high-pressure sodium lamp is a more doubtful case, since it is quite hot (in an alumina tube), while the spectrum has a color temperature of about 2000 K but is far from a black-body spectrum. The spectrum is continuous, and shows very deep self-reversal lines of sodium. On the other hand, a low-pressure sodium arc is definitely luminescent, its light consisting entirely of the bright yellow doublet.
Luminescence occurring while excitation is in progress is called fluorescence. Emission after excitation ceases is called phosphorescence, representing energy stored during excitation that is released later, and which gradually decreases with time. Although this is a useful practical distinction, the two phenomena are closely related and not well distinguished by arbitrarily establishing some dividing time constant, such as 10 ns or 100 μs. A more fundamental distinction is that fluorescence is independent of temperature, while phosphorescence is quenched by an increase of temperature. Why this is reasonable will be explained below, when we discuss mechanisms.
Luminescence occurs in gases, liquids and solids, both amorphous and crystalline. The solid state physicist is principally interested in crystalline materials, for which powerful methods of investigation are available. These materials are also of great practical application, as phosphors that can trace the path of an electron beam, displaying a picture in full color, or convert ultraviolet radiation into visible light, as in fluorescent lamps. A typical cathode-ray-tube phosphor is zinc silicate, Zn2SiO4, the mineral Willemite, activated by Mn, which can be excited by electron bombardment or by ultraviolet, and which fluoresces in a band around 525 nm. It is made by firing 2ZnO + 1.1SiO2 + 0.8 MnCO3 at 1200°C. Organic crystals, such as anthracene and naphthalene that contain only benzene rings, are used in scintillation counters for nuclear radiations.
Chemists are interested in luminescence in organic molecules, as liquids, solutions, or solids. This study began with dyes, which since they absorb light, may also emit it. A chromophore was defined as a molecular group responsible for color. Electrons in such a group make a transition from a singlet ground state to a singlet excited state to absorb radiation. Singlet means that the electron spins are paired to give a zero resultant (antiparallel); such a pair can occupy a single state. There may also be a triplet excited state in which the spins are parallel. These electrons cannot occupy a single state, and must be separated. Because of this difference (the Pauli Principle) the two states do not overlap (are of different symmetry) and a transition between them is unlikely, and called "forbidden." The lifetime of a transition is about τ = 1.5/fν2 seconds, where ν is the reciprocal of the wavelength in centimeters (visible: 25000 cm-1 to 14300 cm-1), and f is about 1.0 for an allowed transition, but 10-6 for a spin-forbidden transition. An allowed transition at about 500 nm has a lifetime of about 4 ns, a forbidden transition about 4 ms.
In a carbon double bond, C=C, it takes about 7 eV to excite an electron from a bonding π level to an antibonding π* level, corresponding to a wavelength of 180 nm. Kicking an electron from one of the unbonded pairs of an oxygen atom to a π* state requires 4 eV, or a 290 nm photon. Exciting an electron in 11-cis-retinal, a molecule important in vision, requires 380 nm in an isolated molecule. In every case, when other atoms are in the vicinity, as in the 6-member hexagonal benzene ring with alternate double bonds, or when the retinal is associated with a protein (opsin), the energy difference is reduced, and the maximum wavelength increased. In the case of retinal, the absorption now occurs in the visual range. Absorption of a photon causes the molecule to straighten out so that it no longer fits in the protein, and this change causes a nerve discharge.
In general, there is a number of excited states, corresponding perhaps to different states of vibration, to any one of which absorption is possible. These states are connected by radiationless processes, which causes the energy to move to the lowest of them in a very short time. This state can then decay to the ground state by an allowed transition, producing fluorescence. The wavelength of the fluorescence will be greater than that of the absorbed radiation (Stokes' Law). In this way, radiation is emitted where absorption is not large. Toluene and benzene fluoresce dark blue when excited by ultraviolet. Anthracene, a colorless crystal, fluoresces blue, the dye methylene blue fluoresces red, and fluorescein (resorcinol phthalein), a fluorescent indicator, which is a yellowish-red powder, fluoresces yellow-green in aqueous solution. There is no relation between the color of a substance and the color of its fluorescence. An indicator is a substance that changes color at a certain value of pH (acidity or alkalinity). In a fluorescent indicator, the color of fluorescence changes.
If a radiationless process connects the excited singlet states with a triplet state at lower energy, energy will pile up in the triplet state and be stored there. When the excitation ceases, thermal agitation may return some energy to the singlet excited state to be emitted, or the triplet state may radiate itself, at its characteristic long time constant, producing phosphorescence. Phosphorescence demonstrates the existence of these metastable triplet states. Phosphorescence is rarely observed in liquids--apparently there is a radiationless mechanism for the de-excitation of the triplet states in this case. The existence of luminescence depends on the absence of a radiationless mechanism for the transition under consideration. Luminescence is unusual, not general.
In gaseous discharges, electron collisions produce new electrons and ions to maintain the discharge, while the excited gas atoms emit their characteristic radiations as they decay to the ground state. Here, the radiation is typically the sharp lines of isolated atoms, so we often see self-absorption and resonance radiation. It is the isolation of the atoms in a gas that makes luminescence possible in this case. In liquids and solids (as well as molecular gases), the radiation is emitted in bands due to transitions between closely spaced levels. Except in a few special cases, the radiation is emitted by isolated levels associated with activators, usually present in very small concentration in a matrix or host material. These luminescence centers can be the activator ion itself, or a distubance of the lattice caused by the activator ion. They correspond to the chromophores of chemists. In the "pure" luminescent materials, such as platinocyanates and uranyl compounds, the centers are ions with unfilled inner electron shells. Solid state physicists have enjoyed studying Tl+ doped KCl crystals in particular.
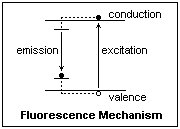 In ZnS and CdS phosphors, activated by Cu, Ag, Au or Mn, the mechanism of photoluminescence is probably as shown in the figure at the right. In these substances, it is associated with photoconductivity, a result of the electron-hole pairs produced by the absorption of photons with energies somewhat greater than the band gap. At the left is shown the ground and excited state of a luminescence center created by an activator ion. These states are localized, and lie in the band gap. When a hole migrates to the vicinity, an electron in the ground state (shown) can recombine with it. The center is now positively charged, and can attract an electron from the conduction band. If the transition is allowed (there is a dipole matrix element), the electron falls to the ground state in around 10-8 seconds. The wavelength of the emission is characteristic of the luminescence center, and is longer than the exciting wavelength. It can be in the visible while the exciting radiation was ultraviolet.
In ZnS and CdS phosphors, activated by Cu, Ag, Au or Mn, the mechanism of photoluminescence is probably as shown in the figure at the right. In these substances, it is associated with photoconductivity, a result of the electron-hole pairs produced by the absorption of photons with energies somewhat greater than the band gap. At the left is shown the ground and excited state of a luminescence center created by an activator ion. These states are localized, and lie in the band gap. When a hole migrates to the vicinity, an electron in the ground state (shown) can recombine with it. The center is now positively charged, and can attract an electron from the conduction band. If the transition is allowed (there is a dipole matrix element), the electron falls to the ground state in around 10-8 seconds. The wavelength of the emission is characteristic of the luminescence center, and is longer than the exciting wavelength. It can be in the visible while the exciting radiation was ultraviolet.
This was called fluorescence, after its occasional but typical occurrence in fluorite, CaF2. The fact that fluorite is not always fluorescent shows that it is not the crystalline matrix, but the impurity, that is responsible. ZnS and CdS phosphors are made by fusing a mixture of the host material, a flux such as NaCl, and the desired impurities. Note that the wavelengths that are absorbed are different from those emitted, so that a luminescence center is transparent to its own radiation, a necessary condition for good efficiency.
When the exciting radiation is removed, the fluorescence rapidly disappears as no new centers are excited, and the excited ones decay exponentially with some time constant τ, as we mentioned above. In some cases, there is an after-glow or phosphorescence with a much slower rate of decay, with time constants from milliseconds to minutes, rather than tens of nanoseconds. Sometimes this is a result of a forbidden (no dipole matrix element) transition, which can give time constants of milliseconds, but which is not fundamentally different from fluorescence. In the more common cases, however, phosphorescence is the result of the presence of a metastable state in the vicinity, for which transitions to the ground state are very improbable.
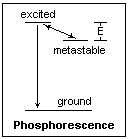 The diagram at the left shows ground and excited states, and also a metastable state an energy E below the excited state. Transitions can occur easily between the excited and metastable states, the differences in energy appearing as lattice vibrations (phonons), as well as between the excited and ground state, with the emission of a photon. While the excitation is active, the excited and metastable states reach an equilibrium. At moderate temperatures, it is more likely that an electron will fall into the metastable state than leave it, because of the activation energy E, so the metastable states generally fill up. When the excitation is removed, the only source of electrons in the excited state are electrons boosted up by thermal energy from the metastable state. When they reach the excited state, they quickly emit a photon and fall to the ground state. This is the phosphorescent radiation, that continues as long as electrons are available from the metastable states. Chemists, with their limited viewpoint, call this "delayed fluorescence," reserving phosphorescence for a direct radiation from the metastable state, but this is not a useful general distinction, because of the variety of mechanisms. The higher the temperature, the more readily the electrons can reach the excited state, and so the shorter the time that the phosphorescence persists. That is, phosphorescence, under this model, is temperature-dependent, in contrast to the previous case with no metastable states, which is temperature-independent. Therefore, fluorescence and phosphorescence are usually distinguished by their temperature dependences, rather than from an arbitrary choice of limiting time constant. As we see, this applies to organic molecules as well as to crystals, though the detailed mechanism is different.
The diagram at the left shows ground and excited states, and also a metastable state an energy E below the excited state. Transitions can occur easily between the excited and metastable states, the differences in energy appearing as lattice vibrations (phonons), as well as between the excited and ground state, with the emission of a photon. While the excitation is active, the excited and metastable states reach an equilibrium. At moderate temperatures, it is more likely that an electron will fall into the metastable state than leave it, because of the activation energy E, so the metastable states generally fill up. When the excitation is removed, the only source of electrons in the excited state are electrons boosted up by thermal energy from the metastable state. When they reach the excited state, they quickly emit a photon and fall to the ground state. This is the phosphorescent radiation, that continues as long as electrons are available from the metastable states. Chemists, with their limited viewpoint, call this "delayed fluorescence," reserving phosphorescence for a direct radiation from the metastable state, but this is not a useful general distinction, because of the variety of mechanisms. The higher the temperature, the more readily the electrons can reach the excited state, and so the shorter the time that the phosphorescence persists. That is, phosphorescence, under this model, is temperature-dependent, in contrast to the previous case with no metastable states, which is temperature-independent. Therefore, fluorescence and phosphorescence are usually distinguished by their temperature dependences, rather than from an arbitrary choice of limiting time constant. As we see, this applies to organic molecules as well as to crystals, though the detailed mechanism is different.
The luminescence efficiency η is the number of luminescent photons per exciting photon, which is always less than one. The luminescence efficiency rises from zero as the concentration of the activator is increased from zero, passes through a maximum at some relatively low concentration, and then declines. The reason for this is that the luminescence centers become closer and closer to one another as the concentration increases, making resonance transfer of their energy increasingly more probable (one decays, the other is excited, with no emission of radiation). Since the energy is now mobile, it can seek out quenching centers where it can be removed without radiation, just as water seeks out a drain.
The idea of creating light directly by electricity has long been a goal. In 1920, it was found that when a DC electric field was applied to a ZnS phosphor in the afterglow, a flash of light was observed. If the field were then suddenly removed, another flash resulted. This Gudden-Pohl effect seems to be caused by emptying of electron traps in the crystal when it is polarized or depolarized. Later, around 1930, it was found that an AC electric field applied to a medium consisting of a phosphor powder, such as zinc sulphoselenide, embedded in a high-permittivity dielectric such as PVC, caused luminescence if the field was high enough. This Destriau effect begins at about 3 kV/cm, but 30 kV/cm is required for a bright display. Apparently the electrons are accelerated sufficiently to excite the luminescence centers in the phosphor. The medium is placed between two plates, one of which is made transparent.
If a sample that has been exposed to the exciting radiation is heated gently, luminescence is sometimes observed, beginning at about 50°C to 100°C. The mineral calcite is known for this. The maximum temperature is kept below about 475°C. Much can be learned from a plot of intensity of phosphorescence as a function of temperature, when a sample is heated at a constant rate. This is called thermoluminescence, though it is not a distinct phenomenon.
Luminescence is also sometimes observed when a sample is crushed, scratched or hammered. This probably is the consequence of the heating of the small particles, and so is just thermoluminescence excited in a different way. Some say it is a piezoelectric effect, but this seems improbable. It is not thermal radiation, of course. Sugar (sucrose) crystals crushed in the dark are triboluminescent. To see this, put some normal granulated sugar in a flat-bottomed bowl. I used a 50 ml beaker to grind them, but a flat-bottomed glass would do. In complete darkness, with dark-adapted eyes, the luminescence is distinctly visible, a whitish glow (dark-adapted eyes have no color vision). Wintergreen life-savers also flash when bitten upon, it is reported. Most minerals that are thermoluminescent also exhibit this triboluminescence, but a few are known for one or the other, but not both. Sphalerite, quartz, magnesite and hemimorphite are triboluminescent but not thermoluminescent, while fluorite, apatite and scapolite are the reverse. A chemical reference even refers to crystalloluminescence, the emission of light upon crystallization, but gives no reference.
It is no surprise that a chemical reaction may leave a product in an excited state which can be de-excited by radiation. For example, O + CS -> CO + S leaves the CO in an excited vibrational state that emits IR when returning to the ground state. The mysterious Will-o'-the-Wisp seems to be a chemiluminescent reaction between phosphine PH3 and hydrogen sulphide H2S produced by decaying organic matter. The small cold greenish flames flash here and there, giving the appearance of rapid, flickering motion. Chemiluminescence and bioluminescence are fascinating subjects, but not very useful as practical light sources, unless you are a firefly looking for sex, or a bat looking for fireflies.
Edmund Scientific sells a kit that includes a chemical called luminol (5-amino-2,3-dihydrophthalazine-1,4-dione), which allows you to observe chemiluminescence in the kitchen. An oxidizer, sodium perborate (NaBO3·4H2O), mixed with sodium carbonate (Na2CO3), is supplied, and also copper sulphate (CuSO4·5H2O), which gives the catalyst Cu++ ions. The perborate mixture seems to be CloroxII®. Hypochlorite bleach or hydrogen peroxide can also be used as oxidizers. Fe++ also catalyzes the reaction, which enables luminol to be used to detect blood (from its hemoglobin). The reaction goes best at pH 11, and is quenched in acid solution. This kit is well put-together, and is worth the money. You get enough luminol for many demonstrations, 5 g. The luminescence is whitish-blue, not very bright, but worth seeing.
Any light observed in nature that appears in the dark with no obvious incandescence or heat is called phosphorescence. The name comes from the element phosphorus, first prepared by Brandt in 1669. When exposed to air, white (yellow) phosphorus, the active allotrope, glows and emits phosphorus trioxide and ozone. This is an example of chemiluminescence, not thermal radiation, though white phosphorus will burst into flame in air. Phosphorescence is usually feeble and greenish, and mysterious. The phosphorescence of the sea is well-known, especially where waves are breaking, when the crests may glow. On an absolutely dark night, this is a magical phenomenon. One source is the flagellate protozoan Noctiluca miliaris that responds to the increase in oxygen in the disturbed water. Luminescence favors the survival of these dinoflagellates since the light betrays their predators to large fish that eat the predators. The dino- comes from Greek for an eddy or whirling, since their flagella whirl. The dino- in dinosaur should really be deino-, coming from the Greek for terrible. So dinosaurs are not whirling lizards. Another is the bacterium Micrococcus phosphoreus. The bioluminescent jellyfish Aequora forskalea contains a substance aequorin that emits visible light when it binds C++ ions. In rotting wood, the honey fungus Armillaria mellea may glow in the dark. The American firefly, and the European glow-worm, Lampyris noctiluca, emit light in their search for sex. Both are actually beetles, the wingless female glow-worm on the ground sought by flying males, or the winged randy fireflies of both sexes in the early summer night. Even the larvae of the firefly are luminescent. All of these are examples of bioluminescence, which is chemiluminescence in an organism. Fireflies use luciferin, which they combine with oxygen from the air, and thereby control the light. There seem to be many examples in the deep sea, where we cannot usually go, and it is otherwise eternally dark. The bark or leaves of the horse-chestnut tree may be steeped to form a fluorescent solution, it has been reported. Quinine water is strongly fluorescent (the quinine, with its ring structure, is responsible). It can be seen to fluoresce in sunlight. Dilute solutions of mercurochrome antiseptic are fluorescent (though this is now hard to find because of the panic over mercury).
Examples and Experiments
It may be an interesting activity to note the light sources that appear in daily life, and classify them as incandescent or luminescent. Formerly, most sources were incandescent, but now luminescent sources are very common. The TV, fluorescent lamps, LED's--even in traffic lights, and the computer screen you are now looking at are all luminescent.
A useful incandescent source is the familiar tungsten-filament lamp. A great deal can be learned about its properties through simple observations with a 25 W straight-filament lamp in a clear envelope. The Philips lamp that I have has a filament about 80 mm long made from a plain cylindrical wire. The lamp should be supplied from a Variac so that the current can be varied, and the intensity made comfortably low. Note the change in color as you bring the filament up to rated voltage slowly. When you can just make out the radiation, it is quite red. It then becomes orange, then yellow, then white as you bring it up. The "white" of an incandescent lamp is not the "white" of the noonday sun, but it appears so to our color perception. I much prefer the yellowish tungsten light to fluorescent light for general illumination. It is, in fact, much whiter than the flames that were once used. (But not whiter than the light of a Welsbach mantle, which is also peculiarly attractive.)
Using an AC ammeter (300 mA scale) and voltmeter, measure the VI characteristic of the bulb. Correlate the appearance of the filament with the current. From these measurements, you can find the resistance as a function of current or voltage. If you plot log R versus log V, you will obtain a very good straight line, showing that the empirical relation R = AVk is well obeyed in the region where the lamp is glowing. I found A = 83.2 Ω and k = 0.41. From this relation, you can predict most of the properties of the lamp. Measure the DC resistance of the cold lamp as well. I found 52 Ω. With 114 V applied, the resistance was 571 Ω, so the resistance increases by a factor of about 11 from cold to operating temperature. This is quite typical of tungsten lamps, and means that there is a current inrush when they are turned on. Incidentally, if you have the ammeter connected when you turn the bulb on, you will blow the fuse in the DMM (I predict).
By comparing the resistance of the bulb at different voltages with the cold resistance, the temperature of the filament can be determined. I found a suitable table on p. E-230 of the 56th edition of the Handbook of Chemistry and Physics, which tabulates the resistivity as a function of absolute temperature. I found that when the lamp is just detectibly glowing red, at a current of 50 mA, the temperature is about 1000 K. At 200 mA, near rated voltage, the temperature is about 2158 K. The filament is serving as a resistance thermometer in this determination. Even at the higher temperature, the peak of the radiation is still in the infrared. The Standard Illuminant A is a tungsten bulb at 2848 K, somewhat hotter than ours, and photoflood bulbs operate at 3200 K (but have correspondingly short lives). If you want to measure the temperature of something incandescent, you can put the filament of the lamp in front of it, and vary the voltage until they are the same color (the filament will seem to disappear). This is the basis of a practical instrument, the optical pyrometer.
We can also estimate the time required for the filament to come up to operating temperature when the bulb is turned on. The specific heat of tungsten is 0.032 cal/g-K, and its density is 19.3. From the cold resistance of the filament of 52 Ω and its length of 80 mm, we can estimate its area at 8.69 x 10-7 cm2, and so its mass at 1.34 x 10-4 g, and its heat capacity at 1.8 x 10-4 J/K (4.184 J = 1 cal). Therefore, to heat the filament by 1885 K from 273 K to 2158 K requires about 0.34 J. If we assume the heating to be at the initial inrush current of 2.3 A and resistance 52 Ω, the time required is about 1.2 ms. This shows us the order of magnitude of the warmup time, which can hardly be greater than a few ms. Induction motors also have an inrush surge when they are turned on, but it takes them much longer to get up to speed. Slow-blow fuses, which are intended for motor loads, will easily handle tungsten loads (even fast-blow fuses may have a delay time that is long enough). A direct measurement could be made using an oscilloscope.
There are a few observations that can be made with the lamp that show that everything is not as simple as it appears. We expect thermal expansion to lengthen the filament by about 1% at rated voltage (about 1 mm), and this loosening is easily seen. The filament then tends to thrash about a little, at a frequency that is obviously unrelated to the power frequency. What causes the motion? In addition to the bright filament itself, you will also see another line of light within the bulb, that is fairly bright and looks like a second filament. If you change your viewing position, you will find that from certain directions it actually appears as two lines, one fainter than the other. If, then, you wet a fingertip and press it on the glass at the proper position, the fainter line will disappear at the same height where you have touched. This shows that the fainter line is a result of reflection from the outer surface of the envelope, while the stronger is a reflection from the inner surface. What you actually see is the real image, which appears to be inside the envelope, of the bright filament produced by the concave surface.
Ultraviolet sources are readily available for observing mineral fluorescence and for erasing EPROM's. Both long- (365 nm) and short- (254 nm) wave sources are available, either with two lamps or with a long-wave filter. These cost from $100 up. There are cheaper alternatives for experiments, which can show you how to apply cold-cathode fluorescents. The best buy is currently All Electronics UVL-2, which has a 113 mm long lamp 15 mm in diameter with reflector, and produces a great deal of UV for only $5.00. You also need a 12V, 500 mA supply, which is easy to find in a lab. The only drawback is that the on time is limited by an internal timer to about one minute, but this is inconsequential. Pressing the button turns it on again. However bad the idea of an "Avon Derma-Spec Skin Imager" might have been, it is an excellent cheap UV source.
Most UV lamps are mercury arcs that run on high-voltage alternating current. The envelope is covered by a visible-absorbing layer that appears dark purple. A small lamp, 50 mm long and 3 mm in diameter, with wire leads at the ends, is available from All Electronics for $8.50 (UV-350), and a suitable power supply for $9.95 (BXA-12529). Both mount neatly on a 3-1/4" x 2-1/2" piece of perfboard (PC-3).
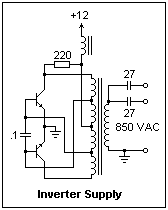 The power supply is a push-pull oscillator running at 20-40 kHz. The reverse-engineered circuit diagram is shown at the right. The operation depends on the details of the transformer, which I do not know, so this circuit is for information only. Measurements while it is in operation may help to understand it better. The output is taken from the secondary of a small transformer, and provides 10 mA at about 850 V. +12 V is applied to terminal 1, and ground to terminal 2 (be very careful to get the polarity correct). There are two output terminals (3 and 4), each coupled through a 27 pF capacitor, and one common terminal (5). Using one output terminal, with the other connected directly with the common terminal, is satisfactory for a 5 mA load (such as the UV-350). The two outputs can be used for two 5 mA loads, one for each output, or a 10 mA load can be connected to the two terminals in parallel. This high-voltage supply is not as lethal as the DC supplies we used for the oscilloscope and photomultiplier, but should still be treated with respect. The absence of large capacitors charged to high voltages is one reason for the relative safety, and the high frequency also helps (skin effect). This little power supply could be made the basis of a low-current DC supply by adding a rectifier and capacitor, but then would become more lethal.
The power supply is a push-pull oscillator running at 20-40 kHz. The reverse-engineered circuit diagram is shown at the right. The operation depends on the details of the transformer, which I do not know, so this circuit is for information only. Measurements while it is in operation may help to understand it better. The output is taken from the secondary of a small transformer, and provides 10 mA at about 850 V. +12 V is applied to terminal 1, and ground to terminal 2 (be very careful to get the polarity correct). There are two output terminals (3 and 4), each coupled through a 27 pF capacitor, and one common terminal (5). Using one output terminal, with the other connected directly with the common terminal, is satisfactory for a 5 mA load (such as the UV-350). The two outputs can be used for two 5 mA loads, one for each output, or a 10 mA load can be connected to the two terminals in parallel. This high-voltage supply is not as lethal as the DC supplies we used for the oscilloscope and photomultiplier, but should still be treated with respect. The absence of large capacitors charged to high voltages is one reason for the relative safety, and the high frequency also helps (skin effect). This little power supply could be made the basis of a low-current DC supply by adding a rectifier and capacitor, but then would become more lethal.
So-called "Blacklite Glow Bulbs" are also available in home-supply stores. These are tunsten filament bulbs, 75 W and 100 W, with a deep-violet visible light filter, and produce feeble UV. An argon lamp is much more useful. Fluorescence in quinine water was scarcely visible, but perhaps this is due to the long-wave nature of what UV there is. They are unsatisfactory as an experimental UV source, but may have some decorative uses. The filament can be seen through the filter. They are certainly the most inefficient light sources that you can actually pay money for.
The Derma-Spec lamp can be used to view fluorescent minerals and other substances. An assortment of fluorescent minerals is available from Edmund Scientifics, but the value of the collection is minimized because none of the samples have any indication of origin or identification, which makes any mineral sample much less desirable. A number of the samples had fluorescent calcite, giving a deep red color. One sample showed brilliant yellow-green fluorescence, probably willemite. The majority of the samples appeared to be of a rather uninteresting but pretty kind with calcite fluorescing dark red and somthing else yellow-green. This collection is not worth more than you pay for it, but it is an available source of fluorescent materials, and worth seeing. With the collection came a paperback The Story of Fluorescence (Middletown CT: Raytech Equipment Company, 1965), by H. C. Wain. This is a pretty good account, meant to accompany a much better fluorescent collection. Raytech still makes UV lamps and accessories; the popular Model 4 is $182. To order, visit Raytech Industries.
The minerals I had around included some that fluoresced. Most minerals do not show fluorescence. A couple of large fluorite octahedra from Southern Illinois gave a very nice blue fluorescence, not strong, but definite. This was, as we have mentioned earlier, the origin of the term fluorescence. An optical calcite rhomb from Saltillo, Mexico fluoresced whitish-yellow, but another from Durango did not fluoresce at all (it was probably too pure). A barite pyramid from South Dakota showed a whitish-yellow fluorescence that looked like a coating on the surface of the tawny crystal. A hexagonal corundum (ruby) crystal fluoresced a deep red. While looking at specimens, it was obvious that paper labels and such fluoresced brightly. There seemed to be fibers that looked like brilliant blue flecks under UV.
An interesting sample was an artifical fluorescent crystal of corundum with an impurity that gave a reddish-violet color in ordinary light. It was labelled "Laser Red Ruby LR-200" and can be obtained at gem and mineral shows. It is, of course, the artificial ruby that is used in optically-pumped lasers, which are an excellent example of fluorescence. The spectroscope showed the sample absorbed strongly from the yellow to the blue, while transmitting a broad band of red. Under UV illumination, the sample fluoresced strongly giving a bright red color, that proved to be a sharp line in the red. This shows very well how the fluorescent color is completely unrelated to the color displayed in white light, though in this case both are red. The red color in white light does not come from anything red in the sample, rather something that absorbs other colors. The red under UV illumination does come from the color centers of the crystal. One make of solderless breadboard fluoresced brightly white-blue, others had no fluorescence at all. The Hitachi scope screen fluoresced a whitish-yellow, different from its color under electron bombardment. The 2BP1 screen did not fluoresce at all; apparently the envelope absorbs all UV. The annular screen of a 6AF6-G electron-ray tube did not fluoresce; instead it appeared dark, as if fluorescing black. I do not know what caused this appearance. Luminol solution is not fluorescent.
A near-IR detector card was available from Quantex, 2 Research Court, Rockville, MD 20850. It is a 2" x 2" square of phosphorescent material, laminated for protection on a 2-1/2" x 4-1/4" card. To "charge" it, it is exposed to near UV--daylight or a fluorescent lamp for a while. When IR falls on it later, charges are promoted out of metastable states to radiating levels, and visible radiation is produced. This is an interesting case of a phosphor with a high activation energy, that is activated by radiation, not thermal motion.
Edmund Scientific sells a UV detector card that shows the intensity of UV radiation that falls on it. There is a bar at the bottom of the card that becomes purple when UV falls on it. This bar is normally bleached white. The darker the colored bar, the more intense the radiation. It suggests holding your sunglass lenses in front of it while exposing it to the sun for 20 seconds. This device was created by Environment Canada. UV is generally easier to detect than IR, since one can make use of fluorescence.
Watches once had luminous dials consisting of a fluorescent material mixed with radium, whose β particles excited it. Radium has disappeared from luminous paint these days, for obvious reasons. If anyone is scared of lead and mercury, he must be terrified of radium. When painting radioactive watch dials, it is best not to lick the brush. Luminous paint must now be phosphorescent, if it has no radioactive stimulation. It would be interesting to see how long the phosphorescence lasts in the absence of excitation. Some watches have electroluminescent lighting, turned on by pressing a button.
References
S. P. Parker, editor, McGraw-Hill Encyclopedia of Physics, 2nd ed. (New York: McGraw-Hill, 1991), article Luminescence.
A. J. Dekker, Solid State Physics (Englewood Cliffs, NJ: Prentice-Hall, 1957), Chapter 16. Any good text in introductory solid state physics should discuss luminescence.
Interesting properties related to luminescence are available from Edmund Scientific, including UV sources, fluorescent minerals, a UV test card, rocks that flash when knocked together, "blacklights," luminous paint, luminous wire, and a chemiluminescence kit.
Return to Electronics Index
Composed by J. B. Calvert
Created 11 April 2002
Last revised 6 May 2002
 In ZnS and CdS phosphors, activated by Cu, Ag, Au or Mn, the mechanism of photoluminescence is probably as shown in the figure at the right. In these substances, it is associated with photoconductivity, a result of the electron-hole pairs produced by the absorption of photons with energies somewhat greater than the band gap. At the left is shown the ground and excited state of a luminescence center created by an activator ion. These states are localized, and lie in the band gap. When a hole migrates to the vicinity, an electron in the ground state (shown) can recombine with it. The center is now positively charged, and can attract an electron from the conduction band. If the transition is allowed (there is a dipole matrix element), the electron falls to the ground state in around 10-8 seconds. The wavelength of the emission is characteristic of the luminescence center, and is longer than the exciting wavelength. It can be in the visible while the exciting radiation was ultraviolet.
In ZnS and CdS phosphors, activated by Cu, Ag, Au or Mn, the mechanism of photoluminescence is probably as shown in the figure at the right. In these substances, it is associated with photoconductivity, a result of the electron-hole pairs produced by the absorption of photons with energies somewhat greater than the band gap. At the left is shown the ground and excited state of a luminescence center created by an activator ion. These states are localized, and lie in the band gap. When a hole migrates to the vicinity, an electron in the ground state (shown) can recombine with it. The center is now positively charged, and can attract an electron from the conduction band. If the transition is allowed (there is a dipole matrix element), the electron falls to the ground state in around 10-8 seconds. The wavelength of the emission is characteristic of the luminescence center, and is longer than the exciting wavelength. It can be in the visible while the exciting radiation was ultraviolet. The diagram at the left shows ground and excited states, and also a metastable state an energy E below the excited state. Transitions can occur easily between the excited and metastable states, the differences in energy appearing as lattice vibrations (phonons), as well as between the excited and ground state, with the emission of a photon. While the excitation is active, the excited and metastable states reach an equilibrium. At moderate temperatures, it is more likely that an electron will fall into the metastable state than leave it, because of the activation energy E, so the metastable states generally fill up. When the excitation is removed, the only source of electrons in the excited state are electrons boosted up by thermal energy from the metastable state. When they reach the excited state, they quickly emit a photon and fall to the ground state. This is the phosphorescent radiation, that continues as long as electrons are available from the metastable states. Chemists, with their limited viewpoint, call this "delayed fluorescence," reserving phosphorescence for a direct radiation from the metastable state, but this is not a useful general distinction, because of the variety of mechanisms. The higher the temperature, the more readily the electrons can reach the excited state, and so the shorter the time that the phosphorescence persists. That is, phosphorescence, under this model, is temperature-dependent, in contrast to the previous case with no metastable states, which is temperature-independent. Therefore, fluorescence and phosphorescence are usually distinguished by their temperature dependences, rather than from an arbitrary choice of limiting time constant. As we see, this applies to organic molecules as well as to crystals, though the detailed mechanism is different.
The diagram at the left shows ground and excited states, and also a metastable state an energy E below the excited state. Transitions can occur easily between the excited and metastable states, the differences in energy appearing as lattice vibrations (phonons), as well as between the excited and ground state, with the emission of a photon. While the excitation is active, the excited and metastable states reach an equilibrium. At moderate temperatures, it is more likely that an electron will fall into the metastable state than leave it, because of the activation energy E, so the metastable states generally fill up. When the excitation is removed, the only source of electrons in the excited state are electrons boosted up by thermal energy from the metastable state. When they reach the excited state, they quickly emit a photon and fall to the ground state. This is the phosphorescent radiation, that continues as long as electrons are available from the metastable states. Chemists, with their limited viewpoint, call this "delayed fluorescence," reserving phosphorescence for a direct radiation from the metastable state, but this is not a useful general distinction, because of the variety of mechanisms. The higher the temperature, the more readily the electrons can reach the excited state, and so the shorter the time that the phosphorescence persists. That is, phosphorescence, under this model, is temperature-dependent, in contrast to the previous case with no metastable states, which is temperature-independent. Therefore, fluorescence and phosphorescence are usually distinguished by their temperature dependences, rather than from an arbitrary choice of limiting time constant. As we see, this applies to organic molecules as well as to crystals, though the detailed mechanism is different.  The power supply is a push-pull oscillator running at 20-40 kHz. The reverse-engineered circuit diagram is shown at the right. The operation depends on the details of the transformer, which I do not know, so this circuit is for information only. Measurements while it is in operation may help to understand it better. The output is taken from the secondary of a small transformer, and provides 10 mA at about 850 V. +12 V is applied to terminal 1, and ground to terminal 2 (be very careful to get the polarity correct). There are two output terminals (3 and 4), each coupled through a 27 pF capacitor, and one common terminal (5). Using one output terminal, with the other connected directly with the common terminal, is satisfactory for a 5 mA load (such as the UV-350). The two outputs can be used for two 5 mA loads, one for each output, or a 10 mA load can be connected to the two terminals in parallel. This high-voltage supply is not as lethal as the DC supplies we used for the oscilloscope and photomultiplier, but should still be treated with respect. The absence of large capacitors charged to high voltages is one reason for the relative safety, and the high frequency also helps (skin effect). This little power supply could be made the basis of a low-current DC supply by adding a rectifier and capacitor, but then would become more lethal.
The power supply is a push-pull oscillator running at 20-40 kHz. The reverse-engineered circuit diagram is shown at the right. The operation depends on the details of the transformer, which I do not know, so this circuit is for information only. Measurements while it is in operation may help to understand it better. The output is taken from the secondary of a small transformer, and provides 10 mA at about 850 V. +12 V is applied to terminal 1, and ground to terminal 2 (be very careful to get the polarity correct). There are two output terminals (3 and 4), each coupled through a 27 pF capacitor, and one common terminal (5). Using one output terminal, with the other connected directly with the common terminal, is satisfactory for a 5 mA load (such as the UV-350). The two outputs can be used for two 5 mA loads, one for each output, or a 10 mA load can be connected to the two terminals in parallel. This high-voltage supply is not as lethal as the DC supplies we used for the oscilloscope and photomultiplier, but should still be treated with respect. The absence of large capacitors charged to high voltages is one reason for the relative safety, and the high frequency also helps (skin effect). This little power supply could be made the basis of a low-current DC supply by adding a rectifier and capacitor, but then would become more lethal.